As a major class of emerging therapies, antibody-drug conjugates (ADCs) already have gained the attention of biopharmaceutical researchers and manufacturers because they combine both the precision of monoclonal antibodies and the potency of highly potent drug compounds. A few ADCs already have entered the market, but many more candidates are progressing through industry pipelines. Platform processes are not yet universal (and it remains to be seen whether they ever will be), but major ADC developers are establishing their own with…
Manufacturing
Change Happens: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (CMC Forum)
In the current global regulatory environment, management and implementation of postapproval CMC changes often can be unpredictable and inefficient. Timelines for change approval can vary from months to years, depending on regional regulatory procedures. Therefore, the challenge in postapproval lifecycle management is to maintain a constant supply of high-quality product while supporting innovation and continual improvement. This was the premise of the CASSS Chemistry, Manufacturing, and Controls (CMC) Strategy Forum held in Gaithersburg, MD, on 20–21 July 2016. The forum…
Polysorbates, Biotherapeutics, and Anaphylaxis: A Review
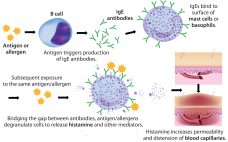
Rapidly increasing use of monoclonal antibodies (MAbs) in the treatment of neoplastic, autoimmune, and inflammatory diseases has led to a dramatic increase in hypersensitivity reactions worldwide, complicating the use of MAbs as first-line therapies and limiting patient survival and quality of life (1). The origins of anaphylaxis are not well understood, though its mechanism is fairly straightforward (Figure 1). It is usually attributed to some undefined intrinsic property or properties of a biotherapeutic — despite the fact that biotherapeutic formulations…
Continuous Processes: Disposables Enable the Integration of Upstream and Downstream Processing
Despite decades of advancement in characterization analytics, biotherapeutics still are largely defined by the manufacturing processes used to make them. This linking of process to clinical results (and thus to commercial success) has made the biopharmaceutical industry somewhat risk-averse when it comes to the adoption of new technologies. That desire to “derisk” biomanufacturing through better process understanding — as well as the need to adapt to uncertainties in patient population size through process flexibility — in turn drives the need…
The Value of Single-Use and Other Flexible Technologies
The biopharmaceutical industry is adding mammalian cell culture capacity at rates that we haven’t seen in over a decade. Over the past five years (2012–2016), we estimate that industry-wide capacity has increased from 3.4 ML to 4.0 ML, an increase of 18% (1). We estimate that industry-wide capacity will increase over the coming five years (2016–2020) to 5.7 ML, an increase of >40%. Clearly, this growth is a response to the continued increase in demand for biopharmaceutical products and to…
Difficult-to-Express Proteins: Resolving Bioprocessing Challenges with a Scalable Perfusion Bioreactor
Recent advances in protein engineering have identified new classes of complex biotherapeutics that challenge existing manufacturing platforms. These products have unique cell culture requirements that make them difficult to manufacture cost effectively. Industry standard bioprocessing platforms include large-scale (1,000–5,000 L) batch and fed-batch stirred-tank bioreactors. Historically, the powerhouse molecule of the biologics industry has been human IgG, which necessitates those large-scale platforms. Difficult-to-express proteins and other new modalities (including precision medicine and orphan drugs) have increased pressure on manufacturers to…
Is Continuous Downstream Processing Becoming a Reality?
Over the past 30 years, several biopharmaceuticals have been produced by continuous cell culture processes run in a chemostat or perfusion mode. In most cases, no alternative was available to produce certain unstable molecules (1). However, downstream processing is and has remained a step-by-step batch operation. Continuous processing generally requires more process knowledge, equipment, and technological advances than do batch processes. With the maturity of bioprocessing and increasing awareness of manufacturing costs, companies are focusing on developing continuous downstream processing…
The Industry’s Hesitation to Adopt Continuous Bioprocessing: Recommendations for Deciding What, Where, and When to Implement
The US Food and Drug Administration has stated its appreciation of continuous bioprocessing (CBP), and some studies have shown that it can save manufacturers time and money. However, the bioprocessing industry is still reluctant to implement continuous bioprocessing right away. It will be interesting to see which companies will be among the first-movers to harness the competitive benefits. Although few biologics today are made using CBP-enabled equipment (e.g., advanced bioreactors), the industry is changing. For biologics already in production, it…
BioPhorum Operations Group Technology Roadmapping, Part 3: Enabling Technologies and Capabilities
Although great strides have been made over the past 20 years to increase the productivity and robustness of manufacturing processes for biopharmaceuticals, the cost and complexity of their development and manufacturing remain high, especially in comparison with those of small-molecule pharmaceuticals. Process improvements are required to increase patient access while maintaining the viability of an R&D-driven biopharmaceutical industry. Facility productivity, cost of goods (CoG), and capital investment all have significant margins for improvement. Such goals can be achieved not only…
Going After a Moving Target: New Production Methods Aid in the Flu Fight
The traditional method of manufacturing vaccines for influenza involves infecting hens’ eggs with the virus, then harvesting and purifying the large amounts of virus that they produce as a result. It’s time-consuming and expensive, requiring large specialized facilities for production. With the advent of genetic engineering and decades of improvement in protein production through cell-line engineering and industrial culture, it was only a matter of time before the vaccine industry saw the real value in modern biomanufacturing instead (1, 2).…