On 21 March 2018, a panel discussion during BPI West in San Francisco addressed ways to improve working relationships between sponsors and contract manufacturing organizations (CMOs). Our goal as organizers was to avoid retreading overfamiliar ground: Many outsourcing articles stress the importance of good communication without delving into actual strategies for improvement. So I asked the panelists to focus on the word better and what that actually means in this context. The most productive way to approach such a discussion…
Manufacturing
Host-Cell Protein Risk Management and Control During Bioprocess Development: A Consolidated Biotech Industry Review, Part 2
Even with increased understanding of host cell proteins (HCPs) and their potential risks, no practical approach has been made available for HCP risk management during bioprocess development. A BioPhorum Development Group (BPDG) team has identified common HCP-related risk factors and built a template for semiquantitative risk assessment during process development based on publicly available information. To this end, the BPDG HCP working team’s assay and knowledge-sharing experts have established a common HCP risk assessment tool and mitigation strategy to guide…
Dual Sourcing of Protein A Resin to Mitigate Supply Chain Risk: A Comparative Study to Determine Equivalence
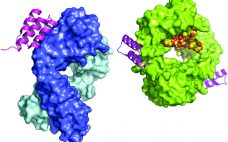
Protein A affinity chromatography is a well-established technology that is used extensively for large-scale purification of monoclonal antibodies (MAbs). With this mode of chromatography, very high product purity can be achieved in a single, relatively simple unit operation. A solution containing the target protein of interest is applied to a liquid-chromatography column at near-neutral pH, and one or more wash steps follow to lower product- and process-related impurities (1). Product is eluted through application of a low-pH buffer. Finally, the…
eBook: SUStainability — Concerning Single-Use Systems and the Environment
Disposable materials have been used in many aspects of biomanufacturing since muromonab was first launched in 1986. Single-use stirred-tank bioreactors first became commercially available from HyClone in 2004 (1). Despite their demonstrated value to bioprocessing, disposable materials remain the subject of wide-ranging differences of opinion. Discussions of any technology are healthy and important for identifying areas for improvement, but some hearsay and bold propositions made regarding single-use components and the environment are not always helpful. Sustainability is an important and…
A Product–Packaging Interaction Study to Support Drug Product Development
Drug packaging is subject to a number of regulatory requirements, including those for product containers and packaging. For example, according to the federal Food, Drug, and Cosmetic Act (FD&C) section 501(a)(3), a drug is considered adulterated “if its container is composed, in whole or in part, of any poisonous or deleterious substance which may render the contents injurious to health.” And 21 CFR states that drug packaging “shall not be reactive, additive, or absorptive so as to alter the safety,…
Large-Volume Wearable Injectors: A New Delivery Approach Could Change the Game
Biologic drugs are a driving force in the pharmaceutical industry. More than 2,700 were in pharmaceutical pipelines as of June 2017 to treat cancers (836), rare diseases (566), neurologic conditions (420), autoimmune disorders (311), and other ailments (567) — triple the number in 2013 overall (1). Biopharmaceuticals make up more than half of all drugs in development and are nudging out traditional small-molecule drugs rapidly while already bringing in billions of dollars in sales. The success of such drugs is…
eBook: The Future of Monoclonal Antibody Manufacturing — Incremental Improvement or Industrial Revolution?
Monoclonal antibody manufacturing is at a crossroads. Biomanufacturers could continue exploring new technologies and fine-tuning proven systems such as mammalian cell expression systems in stirred-tank bioreactor fed-batch cultures. But some experts say an opportunity is arising to turn the industry on its head by taking lessons from other branches of bioprocessing, such as the industrial enzyme sector. Drug makers are criticized often these days for the high prices of their products. The lay media, governments, payers, and patients themselves all…
Facilities of the Future: Intelligent Design and Control Enable Quality, Efficiency, and Good Citizenship
Today’s biomanufacturers need to be able to add capacity and capability quickly; provide increased supply service to customers on demand; and streamline the flows of personnel, traffic, utilities, and materials throughout bioprocess facilities. And companies need to be flexible enough to subtract capacity and retool quickly to produce new or different products. Many future facilities will be automated to some extent and use robotics in manufacturing. With personalized medicine on the rise, bioprocessors can benefit from colocation with academic research…
Flexibility, Automation, and Leadership: Drug-Sponsor Perspectives on Modern Biomanufacturing Facility Design
The title of this featured report — Smart(er) Facilities — came about in conversations with our KNect365 colleagues as they worked to plan this year’s BPI West conference, which took place 19–22 March 2018 in San Francisco, CA. For decades, biopharmaceutical facilities have incorporated cutting-edge designs for supporting processes, products, and human development. Each year, design innovations are rewarded for creating workspaces that facilitate both worker comfort and essential movement of promising drug candidates toward commercialization. In that context, biomanufacturing…
Partnerships for Progress: Supplier Perspectives on Facilities of the Future
Biomanufacturers are constantly tasked with making their products ever more efficiently with ever increasing quality. As major advancements come in biomanufacturing technology, companies find themselves in need of “smarter,” more flexible facilities than ever before. Recently, I asked several industry leaders for their thoughts on some criteria for smarter designs, including why there is such a demand: Cristina Amorim (vice president of facilities, EHS, and sustainability in the life sciences group of Thermo Fisher Scientific) Scott Battist (vice president, general…