Advancements in cell therapy, biofabrication, and synthetic biology have driven the growth of the global regenerative medicine (RegenMed) industry in the past decade. The industry has developed innovative treatment options for patients with otherwise unmet medical needs (1). Human or animal cells or tissues are used as critical raw materials in cell therapy products that can replace, regenerate, or augment patients’ diseased, dysfunctional, or injured cells, tissues, or organs. These cells or tissues can be unmanipulated, or their biological characteristics…
Manufacturing
Spray Freeze-Drying Technology: Enabling Flexibility of Supply Chain and Drug-Product Presentation for Biologics
Biopharmaceutical drug substances (DSs) and drug products (DPs) commonly are stored frozen or refrigerated to maintain stability through long-term storage, handling, and transportation (1). Temperature excursions during storage and transport can affect product quality adversely by compromising the safety and efficacy of these molecules. Thus, cold-chain management throughout the shelf life of these products is a critical component in the supply chain strategy for them. The cost and complexity of cold-chain management is a well-known challenge faced by the biopharmaceutical…
A Multistep Research Protocol to Develop and Implement Validated Guidelines for CMO RFI and RFP Processes: Biopharmaceutical Vendor Evaluation and Selection Minimum Standards (BioVesel)
Pursuant to the proposal for validated minimum standards for biopharmaceutical contract manufacturing organization (CMO) request-for-information (RFI) and request-for-proposal (RFP) processes (biopharmaceutical vendor evaluation and selection minimum standards, BioVesel) (1), we propose herein a multistep research protocol to develop and implement the BioVesel standards. This proposal is intended as a basis for discussion among mulitple stakeholders. Detailed research protocols for each proposed stage in the development and implementation of BioVesel will be drafted and published separately. The context of the proposed…
Development of a Standardized Extractables Approach for Single-Use Components: General Considerations and Practical Aspects — A Manufacturer’s Perspective
The subject of extractables for single-use bioprocess contact materials has been a subject of heated debate since roughly the summer of 2012, when the first ISPE paper was published issuing a call to action to develop a standardized extractables protocol for the industry (1). As a supplier that pioneered the science of extractables (2‒11) and has published extractables data for our products for over 20 years, Sartorius Stedim Biotech (SSB) took the opportunity to look back, take stock, rationalize, and…
Bridging Polymer Science to Biotechnology Applications: A Single-Use Technology Conference Report
Single-use (SU) technology is used more every year throughout the biotechnology industry. As applications now span from cell banking to drug product, that in turn is raising interest in the interaction of extractables with proteins and cells. The second “Single-Use Technologies” conference organized through Engineering Conference International (ECI) was subtitled “Bridging Polymer Science to Biotechnology Applications” and delved into the science of plastics in bioprocessing applications. On 7–10 May 2017 at the Hotel dos Templários in Tomar, Portugal, people from…
Using Data and Advanced Analytics to Improve Chromatography and Batch Comparisons
With all the hype surrounding the industrial Internet of Things (IoT), cloud computing, and digital transformations, the most important information technology factors still are data and the connections of sensors, systems, and applications that generate, store, find, and use those data to obtain operational intelligence. Data volumes are increasing rapidly, and they will continue to do so. The ability to find and make sense of data to obtain intelligence that improves process outcomes is more important than ever. For clinical-…
Rational Selection of Sugars for Biotherapeutic Stabilization: A Practitioner’s Perspective
Biotherapeutics from recombinant DNA technology include diverse modalities such as peptides; enzymes, antibodies, and other proteins; nucleic acids; and cellular therapies. Such products present physical, chemical, and biophysical challenges. Excipients used in stabilization of these biotherapeutics can be broadly classified into the following classes (subgroups) that have been reviewed carefully elsewhere (1–4) : Buffers (e.g., phosphate, acetate, and histidine) Tonicity agents/stabilizers (sugars such as sucrose, polyols such as sorbitol) Bulking agents (lyoprotectants such as mannitol) Surfactants (e.g., polysorbates) Antioxidants (e.g.,…
Computational Science Changes Biolaboratory Design
Until relatively recently, life-science research was characterized by test tubes, Petri dishes, and centrifuges. Now, as with many industries, the life sciences are undergoing a digital transformation. Computational science is changing laboratory design. The healthcare industries always have generated large amounts of data. What has changed is the available information technology. With the growth of cloud computing, large data sets — and the high-speed tools for analyzing them — are available increasingly to a degree not possible with traditional servers…
eBook: Scalable Cell-Based Immunotherapy Manufacture: A Comparison of Single-Use Agitated and Static Expansion Technologies
Early clinical results indicate that personalized autologous immunotherapies could revolutionize cancer treatment (1). However, challenges lie in the realization of cost-driven, scalable cell therapy (CT) manufacturing strategies (2) for generating sufficient therapies to treat a populace, thereby limiting their translation to public health (3). Primary challenges involve complex needle-to-needle logistics, complexities in closed processing, and high variability in starting cell materials that define the autologous nature of such therapies. Despite barriers in industrial-scale manufacture, public health management already has engaged…
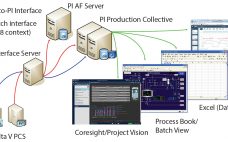
Big Biotech Data: Implementing Large-Scale Data Processing and Analysis for Bioprocessing
Managing large amounts of data presents biopharmaceutical companies of all sizes with the need to adopt more efficient ways to handle the ongoing influx of information. At KNect365’s September 2017 Cell and Gene Therapy conference in Boston, Lisa Graham (founder and chief executive officer of Alkemy Innovation, Inc. in Bend, OR) spoke about the need for data management, data analysis, and process monitoring systems to evolve. Although she was speaking at a cell therapy event, her points are applicable to…