Quality by design (QbD), risk management, and new technologies are shaping biologics formulation work in the 21st century. We saw much evidence of this at the BioProcess International Conference and Exhibition in Boston last fall, where a wide range of talks filled the Drug Product, Fill–Finish, and Formulations track during the week after Labor Day. Dingjiang Liu (Regeneron) offered a high-level discussion from the BioPhorum Development Group (BPDG) on “An Intercompany Perspective on Biopharmaceutical Product Robustness Studies.” Such studies ensure…
Manufacturing
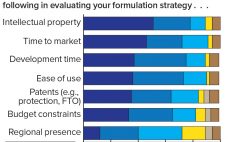
Early Stage Development of Advanced Formulations in the Drug Development Process Provides Competitive Advantages: Survey Predicts That Drug Product Formulation Recognition and Budgets Will Increase Significantly
New antibody formats and aggregate-prone, subcutaneously administered protein therapeutics present biopharmaceutical companies with major challenges regarding protein stability and aggregation. At the same time, protein stability often is not given enough attention in early stages of development. Protein aggregation reduces drug activity so that increasing doses are needed to achieve the same desired effect. Even worse, protein aggregates can induce immunogenecity that endangers patients and compromises product approval. A market study presented for the first time at the Bio-Europe 2018…
Manufacturing Insights: Interviews from BWB 2018 Highlight Perspectives on Streamlining Manufacturing Timelines
Addressing manufacturing and technologies strategies to accelerate market entry is one of BPI’s highlighted themes for 2019. In partnership with our conference colleagues in Informa’s KNect365 division, this already has been a shared theme, reflecting the general goals of the industry and related advice from its regulators. BPI’s summer 2018 preconference ebook included interviews by Dan Stanton (editor, BioProcess Insider) with speakers previewing their talks for the BPI Conference in Boston on 7 September 2018. Two of those conversations focused…
Aspects of Acceleration: Biomanufacturers Need Smart Strategies to Speed Products to Market
No matter what the industry, it’s widely accepted that slow-moving companies give their nimbler competitors an advantage, allowing them room to dominate the market even if their products are not superior. “Me-too” products and their sponsors often are seen as followers rather than leaders — even if they offer improvements over what is already available. Fast movers are flexible and adaptive to a dynamic business environment. They capitalize on opportunities and navigate risks and challenges by responding quickly to changes…
Worldwide Biopharmaceutical CMO Capacity Analysis
Contract manufacturing organizations (CMOs) are a core part of the biopharmaceutical industry, with commercial manufacturing making up about a third of marketed products. Here we examine worldwide CMO biopharmaceutical manufacturing (bioprocessing) capacity, concentrating on “mainstream” CMOs — defined as those providing primarily mammalian cell culture or microbial fermentation services for manufacture at any scale of proteins and antibodies. This analysis follows our recent article examining worldwide bioprocessing capacity status and trends (1). Survey Methodology The 2018 15th Annual Report and…
Development of Large-Scale Bulk Freezing Systems
The biopharmaceutical industry is under pressure to generate value for shareholders and find innovative therapies while driving down development and manufacturing costs. As the industry considers more flexible and scalable production solutions for patients, new therapies will continue to be developed faster than ever before. These products will need to rely on robust and reliable manufacturing solutions. To deliver on that challenge, streamlining manufacturing processes will be necessary so that many lots of drug substances can be manufactured, stored, and/or…
The Changing Landscape of Single Use in Bioprocessing: An Interview with Stefan Schlack and Jean-Marc Cappia
Single use in bioprocessing has changed significantly in recent years. To find out how a leading supplier to the biopharmaceutical industry is redefining its technology to align with new market challenges, science writer, Sue Pearson, had the opportunity to interview Stefan Schlack, Head of Bioprocess Marketing, and Jean-Marc Cappia, Head of Segment Marketing Vaccines, both at Sartorius Stedim Biotech (SSB) in Goettingen, Germany. Redefining Single Use Pearson: Sartorius Stedim Biotech is a recognized leader in single-use technology, and you’ve recently…
Messenger RNA Drugs: Engaging the Machinery of Patients’ Cells to Therapeutic Effect
Although most of the bioprocess industry has focused on process development for large-molecule formulations (e.g., protein drugs), a growing segment of the industry has been concentrated on other types of biotherapeutics to leverage advances in understanding of immunology and genetic engineering. Such technologies may emerge both as tools for drug manufacturing and at some point, as biopharmaceuticals, biotherapeutics, vaccines, and cell and gene therapies,  themselves. What brings mRNA research to BioProcess International’s attention is the increasing interest turned toward therapeutic…
Development and Biomanufacturing Strategies for Next-Generation Antibody-Drug Conjugates
The development and manufacture of antibody-drug conjugates (ADCs) requires a series of complex steps. ADC manufacturers must comply with guidelines for both the small-molecule linker-drug and the monoclonal antibody (MAb). The authors describe their company’s development of its lead ADC product. They review process decisions, including the issues that factored into their selection of single-use systems, manufacturing challenges (differences between ADCs and MAbs), and testing methodologies for extractables and leachables. Synthon began as a small-molecule generics company in 1991. During…
Introduction: The Ins and Outs of Market Demand
From transport and holding of bulk drug substance to shipping, warehousing, tracking, and distribution of final packaged drug products, biopharmaceutical supply chain logistics can be described as an industry in itself. And that’s just one side of the story. Even though much of the work of establishing and maintaining supply chains might happen outside the manufacturing environment, all organizations that develop processes and final products depend on having the raw materials and available components and resources to do their work.…