As cell therapies move through the clinic toward commercialization, respondents to an Informa Connect industry survey are beginning to look to allogeneic — or off-the-shelf — products as “the next big thing.” Almost 200 people contributed to the Cell Therapy Analytics Report, revealing their current positions within the burgeoning cell and gene therapy space and offering their thoughts and predictions for the future. Most survey respondents work within companies developing oncology products. Of those, the largest group (41%) said that…
Manufacturing
eBook: Viral Vectors for Vaccines — A Virtual Conversation on Production and Analysis
Although today’s vaccines are safer, more effective, and more accessible than they were even 20 years ago, the emergence of new, complex pathogens has exposed limitations in traditional vaccine strategies. Viral vector vaccines (VVVs) hold great promise for confronting those now-intractable pathogens. Combining the best features of live-attenuated and DNA-vaccine approaches, these next-generation prophylactics seek to harness the infectivity of non- or low-immunogenicity viruses to shuttle antigen-encoding DNA from target pathogens into host cells. The resulting transduced cells then initiate…
The Proof Is in the Data: Extractables and Leachables
Extractables and leachables (E&Ls) must be addressed in material and process validation programs. Extractables are compounds that can be extracted from a material in the presence of solvents with varying polarity under extreme conditions. Materials manufacturers should make extractables guides available to end users. Leachables are compounds that migrate from a material in the presence of an actual formulation under normal process operating conditions. Extractables information can be helpful as a basis for evaluation of potential process-equipment–related leachables (PERLs)testing. However,…
eBook: Biologics Stability — Lifecycle Management of Drug Products
The biomanufacturing industry’s increasing attention to risk mitigation through quality by design (QbD) and the emergence of complex therapeutic modalities have driven the need for a lifetime-management approach to assuring drug product stability. To that end, industry guidelines have been (or are being) developed to guide the industry toward a “holistic approach” to conducting stability assessments. However, not all methods are stability indicating, and many more industry concerns need to be addressed. This BPI eBook offers perspectives on ICH Q12…
The Value of Plug-and-Play Automation in Single-Use Technology
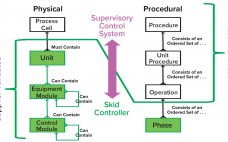
Automation can improve efficiency, track performance, adjust operations, and liberate operators from mundane routines. Automation requires a flexible set of tools that align well with the inherent flexibility of single-use technology (SUT). Although SUT flexibility enhances a biomanufacturer’s ability to modify operations to meet the needs of today’s dynamic industry, it also increases timelines and costs related to customizing and validating automated additions. We present herein the findings of a team of industry automation experts who are sharing their experiences…
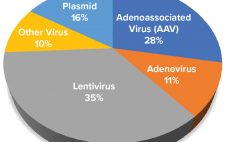
Capacity Analysis for Viral Vector Manufacturing: Is There Enough?
Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel,…
Risk and Lifecycle Management for Biopharma Operations
By working with the best biopharmaceutical companies for over a decade, 4Tune Engineering (4TE) has built a portfolio of services that enables companies to address current-century challenges. The biotechnology industry needs to address advanced therapies and personalized medicines and deliver explicit patient outcomes. Biologics today fall into four categories: monoclonal antibodies (MAbs), biosimilars, advanced therapeutic medicinal products (ATMPs), and cell and gene therapies (CGTs). Consequently, we can ask whether our manufacturing science and technology (MSAT) approaches are up to the…
AAV Vector Manufacturing Platform Selection and Product Development
Adenoassociated virus (AAV) vectors have emerged as the prominent delivery mechanisms of corrective gene therapies. Three such products — Glybera (alipogene tiparvovec, uniQure), Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), and Zolgensma (onasemnogene abeparvovec-xioi, AveXis) — have been licensed, and a growing number of candidates are entering late-stage development. In mapping out an AAV gene therapy product development strategy, biomanufacturers should address fundamental considerations for their manufacturing strategies for both phase 1–2 clinical evaluation and translation for commercial market supply. A manufacturing…
The Road to Commercialization: A Commercial CDMO’s Perspective
Richard Richieri, chief operation officer (COO) of Avid Bioservices, recently presented an Avid case study and strategy to design, prepare, and execute process validation in preparation for successful product approval inspections. The goals of the presentation were to share lessons about some of the strengths learned from Avid’s experience and to offer advice on industry best practices. Finding a CMO that meets your quality expectations and scale is a key driver for your eventual commercial success. Particularly, working with CMO…
Launch of the First Vaccine Bioprocess Training Program: A Standardized but Flexible Course to Boost the Global Vaccine Industry
Based on the many forms that modern vaccines can take, their manufacturing is complicated. Unlike monoclonal antibodies (MAbs), vaccine manufacturers have no “template” platform to follow. Most vaccine producers develop their manufacturing processes from scratch, a prospect that can be challenging for small to mid-sized companies. Bioprocessing is the key challenge in vaccine manufacturing. Without a well-developed and understood process, a manufacturer will face serious challenges in commercial production: e.g., low yields, high costs, and difficulties in meeting quality standards.…