Managing risk in single-use systems design and implementation is a shared responsibility. The ultimate responsibility for drug processes and products will always remain with manufacturers. However, implementation of single-use systems can shift responsibilities to suppliers within key areas, including design and sterilization, which must be clearly controlled and validated. This Special Report discusses how suppliers and manufacturers when working together can mitigate the risk of applying single-use systems in biopharmaceutical production from design through validation to point-of-use testing and operator…
Manufacturing
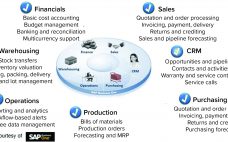
Challenges and Benefits of Networking Process Control Manufacturing Systems: Integration to Business Systems in Industry 4.0
Networking of manufacturing process control systems can lead to benefits of efficiency, increased productivity, and better facility use, leading to lower cost of goods (CoG). Furthermore, integration of manufacturing systems with manufacturing execution systems (MESs) upward to an enterprise resource planning (ERP) business system improves overall organizational efficiency. An ERP system enables optimization of intrafacility manufacturing resources. For multiple manufacturing facilities, it facilitates optimization across a company‚Äôs manufacturing network. Enabling Technologies and Systems At the enterprise resource planning level (Figure…
Designing the Right Strategy for Digital Transformation: How a Pragmatic Approach to Digital Transformation Can Help Biomanufacturers Adapt to a Challenging Future
Although the biopharmaceutical industry has enjoyed explosive growth over the past three decades, it still faces an assortment of challenges. Those include growing portfolio complexities, increased demand volatility, stringent regulatory requirements, increased pricing pressures, and growing technological complexities, all leading to severe pressure on profit margins. To overcome such pressures, biopharmaceutical operations need to become more reliable and agile, and they must realize efficiency gains in both manufacturing and supply chains. Digital transformation offers strong value opportunities, including a potential…
Gene Therapy Trends and Future Prospects
Gene therapy is defined as the transfer of genetic information to a patient for treatment of a disease. Clinical investigation of such therapies began in 1990 with a treatment for a rare immunodeficiency disorder and since has expanded to almost 1,000 clinical studies in 2019 (1, 2). In its most straightforward incarnation, the goal of gene therapy for genetic diseases is long-term expression of a transferred gene at levels that are high enough to be therapeutic, an approach sometimes called…
Cell and Gene Therapies Get a Reality Check: A Conversation with Anthony Davies of Dark Horse Consulting Group
As founder of cell and gene therapy (CGT) specialist firm Dark Horse Consulting Group in California, Anthony Davies speaks from a quarter century of experience including former positions at Onyx Pharmaceuticals, Syrrx, ZymeQuest, Serologicals, Geron Corporation, Capricor, and 4D Molecular Therapeutics ‚ÄĒ and he currently serves on the board of directors for TrakCel and the scientific advisory boards for Akron Biotech and BioLife Solutions. In his plenary address at the Phacilitate 2020 Leaders World conference (part of Advanced Therapies Week…
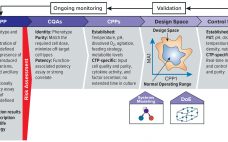
Quality By Design for Advanced Therapies: An Informed Route to Enhanced Late-Stage Clinical Success and Empowered Process Flexibility
As advanced therapies, including regenerative medicines, progress toward commercialization and market approval, early warnings from key opinion leaders (1, 2) regarding the importance of better understanding quality target product profiles (QTPPs) and critical quality attributes (CQAs) of such products have resounded ever louder (see the ‚ÄúTerminology‚ÄĚ box for definitions). Costly late-stage delays, redirections, and even abandonment of clinical programs can be linked to quality issues associated with inadequate understanding of process and product. Therefore, a review of the benefits of…
Transforming Personalized Medicine into Off-the-Shelf Cell Therapies
Initial progress in cell and gene therapy has seen 12 advanced therapeutic medicinal products (ATMPs) become available on the market in 2019 for a range of conditions, from monogenic diseases to cancer. Despite such progress, development of clinically and commercially successful cell therapies presents manufacturability challenges and questions about bypassing patients‚Äô immune systems. The availability of rapid sequencing and next-generation bioinformatics has made it possible to understand the mechanisms of disease better and accelerate development of therapeutic responses. The same…
Sharing Viral Vector Expertise: A Conversation with Yposkesi’s Chief Executive Officer
As a full-service contract development and manufacturing organization (CDMO) specializing in gene therapy development, Yposkesi produces recombinant adenoassociated virus (AAV) and lentivirus (LV) vectors using adherent-based and suspension-adapted cell expression platforms. Alain Lamproye joined the company as chief executive officer (CEO) in January 2017, having served previously as president of the biopharmaceutical business unit of Novasep (2012‚Äď2017) and as CEO of Henogen, its subsidiary dedicated to gene therapy. He has held managerial positions in pharmaceutical operations at Merck Serono (including…
Ask the Expert: Key Considerations for Cryogenic Preservation and T-Cell Viability
Cryopreservation provides critical protection for cell therapies by minimizing genetic changes. But cooling too slowly or quickly risks diminishing cell viability upon thaw. On 11 March 2020, Peter Kilbride (senior research scientist) and Julie Meneghel (cryobiologist), both of Cytiva (formerly GE Healthcare Life Sciences), discussed the importance of controlled-rate cryopreservation. Illustrating how mammalian cells change when frozen, Kilbride and Meneghel offered concrete cryopreservation strategies and identified temperatures at which it is safe to stop controlled cooling and transfer drug product…
A Healthy Biosimilars Market Promotes Innovation and Affordability
Innovative drug manufacturers require an opportunity to recoup capital and opportunity costs that they incurred to develop new medicines. Once patents have expired, competitors should be empowered to promote widespread affordability. An exclusivity period followed by a competitive market would promote the otherwise incompatible objectives of incenting innovation and promoting affordability. That careful balance exists for small-molecule medicines but not yet for biologics. The innovation side of the biologics market is working as intended, but a robust market for lower-cost…