Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
Downstream Processing
A Two-Step Purification Process: Application of HIC Membrane Chromatography in a Disposable 2,000-L Clinical Facility
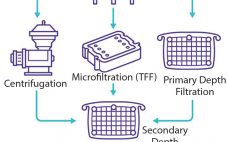
Given paradigm shifts in the biopharmaceutical industry over the past decade, product development timelines are squeezed as the number of molecules entering clinical development continues to increase. Manufacturing facilities, especially those supplying clinical trial materials, have had to adapt to this trend. One popular approach is to have fully disposable equipment that allows for quick product changeover and flexible manufacturing capacity to respond to variable clinical demand. Although many facilities-related technologies exist to support that concept (e.g., disposable probes and…
IgG Purification By Ultrafiltration: Time for Another Look
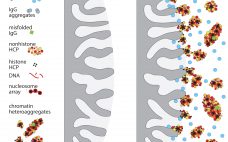
One of the early disappointments in development of immunoglobulin G (IgG) purification technology was ultrafiltration on membranes with 50–100 kDa cutoffs. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) showed that most host cell proteins were smaller than that. IgG was retained. Parallel concentration and buffer exchange could be performed going into a follow-on polishing step. These features made it an obvious candidate for initial capture, but it did not perform as hoped. Membrane fouling sabotaged its concentration–diafiltration potential, and prohibitive…
Large-Scale Purification of Factor-IX: Comparing Two Affinity Chromatography Resins
Human clotting factor-IX (F-IX) is a glycoprotein that is essential for normal hemostasis (1). A deficiency of F-IX in human plasma is caused by an absence or functional mutation of the F-IX gene that expresses inactive F-IX in plasma. That leads to hemophilia B (“Christmas disease,” named after its first identified patient), a genetic disorder in which the blood-clotting cascade is disturbed (2, 3). The structure and amino-acid sequence of F-IX are similar to those found in other vitamin-K–dependent glycoproteins.…
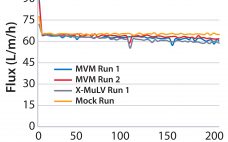
Advanced Viral Clearance Study Design: A Total Viral Challenge Approach to Virus Filtration
Biologics derived from mammalian organisms have been accepted for therapeutic use for almost a century (1). However, these pharmaceuticals have the potential for contamination with pathogenic adventitious agents such as viruses. With cell-line–derived recombinant proteins, the viral risks commonly include viruses in the Retroviridae and Parvoviridae families (2). As patient safety and manufacturing facility suitability became significant concerns in the 1980s and 1990s, several industry and regulatory bodies reached consensus on how to approach the unique challenges of viral safety…
Evaluating Adsorptive Filtration As a Unit Operation for Virus Removal
Most recombinant monoclonal antibodies (MAbs) are produced by mammalian cells. Because biopharmaceuticals derived from mammalian tissue culture carry the risk of adventitious virus contamination, regulatory agencies expect risk-mitigation strategies to include validation of purification unit operations for their ability to clear viruses (1). Guidelines from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) describe how to prove viral clearance in downstream purification processes using an orthogonal approach (2). Viral log10 reduction values (LRVs) are…
New Dimensions in Single-Use Filtration
Whether viral vectors are clarified or the bioburden after cell harvest needs to be reduced to recover antibodies, such applications in biopharmaceutical production require large filtration areas. Single-use technologies are indispensable in many such bioprocesses. Although some single-use filter assemblies have reached their limits, Sartorius Stedim Biotech has made developments to revolutionize these production steps. Scale-Up Limitations in Single-Use technology Conventional stainless steel process systems have been established for decades in the pharmaceutical industry. They are the basis of safe…
Establishing Effective High-Throughput Contaminant Removal with Membrane Chromatography
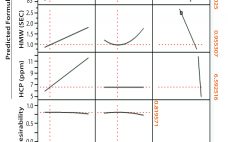
Bharat Serums and Vaccines Limited (BSV) in India conducted a study based on effective removal of host cell proteins (HCPs) from a recombinant hormone with a wide isoform profile in the acidic range imparting drug-product activity. Because the hormone and HCPs have a similar range of active species, the purification process with conventional chromatography resins had difficultly removing those HCPs from the active isoforms of the hormone. To solve that issue, a membrane chromatography technique was implemented. Our initial choice…
Streamlined Column-Packing Design for a New Commercial Launch Facility
To meet network demand for a commercial launch facility, Genentech (Roche) designed a new downstream train and built it within an existing building shell at the company’s Oceanside, CA, site. This downstream train included new technologies to allow for rapid technology transfer of different new products in the company’s drug pipeline. One technology that was pursued was the Axichrom column platform from GE Healthcare and associated column packing equipment to streamline column packing design. Here we focus on how a…
Addressing the Challenge of Complex Buffer Management: An In-Line Conditioning Collaboration
Read this article from scientists at Cytiva (GE Healthcare at the time of publication) to learn more about buffer preparation strategies now. Preparation and storage of buffers is a challenge for biopharmaceutical companies developing protein-based pharmaceuticals. The need for volumes of buffer to purify increasing upstream titers have become a major bottleneck in biopharmaceutical downstream processing. Italian biopharmaceutical company Kedrion Biopharma collects and fractionates blood plasma to produce plasma-derived therapeutic products for treating and preventing serious diseases, disorders, and conditions…