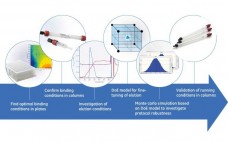
Virus filters are used in biomanufacturing to ensure the safety of biopharmaceutical drug products. As part of filter implementation, manufacturers are required to validate that the filtration process can indeed remove virus. Validations are typically performed at contract testing organizations (CTOs) that are “equipped for virological work and performed by staff with virological expertise in conjunction with production personnel involved in designing and preparing a scaled-down version of the purification process” (1). Virus removal capability of a filtration process is…
Downstream Processing
Building and Operating a Single-Use Facility: Reducing Time, Cost, and Risk
This podcast features: Richard Pearce, Director of Strategy and Business Development, EMD Millipore [sc_embed_player_template1 fileurl=”http://commondatastorage.googleapis.com/bpidm/Single-Use/TASCAM_0052.mp3″] This podcast was recorded at the 2013 BioProcess Theater which took place at the 2013 BIO International Convention, April 22-25, 2013. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business and…
Processing Biologics Using a Disposable Production Train?
This podcast features: David J. Kenyon, PhD, Senior Director, Process Services, Gallus BioPharmaceuticals, Inc. [sc_embed_player_template1 fileurl=”http://commondatastorage.googleapis.com/bpidm/Single-Use/TASCAM_0067.mp3″] This podcast was recorded at the 2013 BioProcess Theater which took place at the 2013 BIO International Convention, April 22-25, 2013. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business…
Purification and Concentration of Nanoparticles using Single-Use TFF
This podcast features: Jim Simmons, Central US Sales Manager, Spectrum Laboratories, Inc. [sc_embed_player_template1 fileurl=”http://commondatastorage.googleapis.com/bpidm/Single-Use/TASCAM_0050.mp3″] This podcast was recorded at the 2013 BioProcess Theater which took place at the 2013 BIO International Convention, April 22-25, 2013. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business and scientific…
Process Evolution from the Iron Age to the New Age – A Transition from a Stainless Steel and Glass to a Full Disposable Upstream Process
This podcast features: Michael Brown, Senior Associate Scientist, Avid Bioservices, Inc [sc_embed_player_template1 fileurl=”http://commondatastorage.googleapis.com/bpidm/Single-Use/TASCAM_0051.mp3″] This podcast was recorded at the 2013 BioProcess Theater which took place at the 2013 BIO International Convention, April 22-25, 2013. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business and scientific trends…
Accelerating BioProcess Development Using Microbioreactors
This podcast features: Frank Kensy, Managing Director, m2p Labs [sc_embed_player_template1 fileurl=”http://commondatastorage.googleapis.com/bpidm/Single-Use/TASCAM_0066.mp3″] This podcast was recorded at the 2013 BioProcess Theater which took place at the 2013 BIO International Convention, April 22-25, 2013. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business and scientific trends impacting the…
ROUNDTABLE: Flexible Facilities – Strategies for Supplying Complex Biologics in a Changing Global Market
This podcast features: Chairperson: Susan Dexter, Principal Consultant, Latham Biopharm Group Speakers/Panelists: Geoff Hodge, VP of Biomanufacturing Services, Xcellerex, a GE Healthcare Company Dr. Abdullah Baaj, Founder and CEO, Boston Oncology Dr. Yan-Ping Yang, Head of Downstream Processing, Sanofi Pasteur Peter Cramer, Vice President, M&W Group[sc_embed_player_template1 fileurl=”http://commondatastorage.googleapis.com/bpidm/Single-Use/TASCAM_0053.mp3″] This podcast was recorded at the 2013 BioProcess Theater which took place at the 2013 BIO International Convention, April 22-25, 2013. The BioProcess Theater, a 50-seat amphitheater located on the exhibition…
Polishing of Monoclonal Antibodies Using Captoâ„¢ MMC ImpRes in Bind and Elute Mode
GE Healthcare Life Sciences MAb purification toolbox employs protein A chromatography media (resins) such as MabSelect SuReâ„¢ or MabSelect SuRe LX, in the capture step. After the initial protein A step, there is a range of options for intermediate and polishing purification steps. One option is Capto MMC ImpRes, a new chromatographic medium based on a multimodal cation exchange ligand. This work describes a rapid procedure to establish a robust second step in bind/elute mode for the purification of a…
Broadening the Baseline
When the editors of BPI asked us at BPSA to put together a content-rich article for the single-use supplement, we were happy to do so. Our challenge was how to bring in multiple viewpoints about the growing business of single-use that would be a “quick read” for the BPI audience. The answer: an expert colloquy (a “conversational exchange or topical dialogue”). Represented here are several of the most qualified industry spokespersons in single-use — all are members of BPSA and…
Single-Use Pumps Take Center Stage
The multibillion-dollar global biopharmaceutical industry is placing increased emphasis on development and manufacture of advanced biologics. Such products offer exciting potential for the development of drugs that could provide as-yet-unknown treatments for a wide array of diseases. One important goal is to commercialize biologic products as early as possible within the typical 20-year patent window. Patent submission must occur during drug development. Much work follows a patent filing, including further product development, toxicity checks, and clinical trials. Hopefully, US Food…