In a vaccine development program, the probability of success at each transition decreases, even though the actual probability of moving from one phase to another can be 50–80% (Figure 1). Many compounds and vaccine candidates are screened out even before they get into preclinical studies. Developers can implement different approaches to reduce product failure risk before a program gets expensive, including Establishing a product development plan (PDP) Identifying and mitigating risk with gap analysis Learning from the mistakes of others…
Downstream Processing
Industry Adoption of Membrane Adsorbers
Membrane adsorbers (MAs) are the fastest-growing segment in single-use bioprocessing. But their future is not entirely certain. According to BioPlan Associates’ latest survey of biopharmaceutical manufacturing, the MA market has been growing at ~20% annually since 2006 (1). Paradoxically, however, the segment may not be a true “rising star.” Our study also shows that MAs remain among the least-often adopted devices among biomanufacturers. So the question of how and whether MA technology can revolutionize bioprocessing remains open. Market for Membrane…
Simpler and More Efficient Viral Vaccine Manufacturing
Human and veterinary vaccines are divided into five main categories: conjugate, toxoid, subunit, inactivated (killed), and live (attenuated) vaccines (1). The vast majority of currently licensed human and veterinary vaccines are inactivated or live (2, 3). They are produced mostly using adherent cells: primary cells such as chicken embryo fibroblasts (CEF), human diploid cells such as MRC-5, or continuous cell lines such as Vero and MDCK (4). The pioneering legacy inherited by vaccine manufacturing development has led to strategies for…
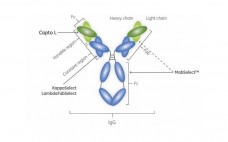
Emerging Challenges to Protein A
Protein A affinity chromatography has been a target for replacement since its commercial debut, mainly because of its high acquisition cost. The technique became established despite the cost because it was born into an industrial culture that favored speed to market over manufacturing economy (1). Vendors have since strengthened protein A’s position with incremental but worthy improvements such as higher capacity, lower ligand leaching, and modest tolerance of NaOH. Collateral improvements in polishing technologies, such as the high throughput and…
A Platform Approach for the Purification of Domain Antibodies (Dabs)
A three-step purification process for a Dab successfully developed and verified. The step yield ranged between 86% and 99%, giving a total process yield of approximately 81%. The ECP in the start sample was more than 200 000 ppm whereas the final sample contained only 5.5 ppm. The endotoxin content in the feed was approximately 2 million endotoxin units/mg of protein and the final sample was below the limit of quantification. Protein L leakage was undetectable in all samples. This…
MFI for Particle Characterization of Biopharmaceuticals Today
Micro-flow imaging (MFI) is a sensitive, simple and automated method for the analysis of sub-visible particles and translucent protein aggregates that provides particle size, count and morphology. Because it quantifies particle shape, this technology can discriminate groups of particles from each other, and evaluate how these groups change over time. In this article, we provide an overview of how biopharma is using MFI to characterize and monitor their protein formulations in new and emerging applications.
POROS® CaptureSelect™ Affinity Columns for Rapid, Small-Scale Purification and Sample Preparation of Recombinant Proteins
This application note describes a model system in which Enbrel® protein, a fusion protein consisting of TNF receptor and IgG1 Fc, is purified from a dilute sample matrix for further analytical characterization. The resulting purified protein was subjected to N-linked glycan structural analysis by mass spectrometry (MS) and its aggregation state was assessed by analytical ultracentrifugation (AUC). This data set serves as a model to demonstrate the capability of the POROS® CaptureSelect™ product line for the affinity purification of biomolecules…
Enlightening Results
Separating spectroscopy from spectrometry is not as straightforward as it might seem. Spectroscopy is the science of the interactions between matter and radiated energy, and spectrometry is the technology that applies that science (1). The former generates no results on its own. It is concerned with spectra produced when matter interacts with or emits electromagnetic radiation, including all methods of producing and analyzing light spectra using spectroscopes, spectrographs, spectrometers, and spectrophotometers. The distinction should come from the meanings of the…
Protein A
The number of blockbuster monoclonal antibody (MAb) drugs continues to grow. In 2008, MAbs generated revenues in excess of US$15 billion (1), making them the highest-earning category of all biotherapeutics. The world MAb market will reach $62.3 billion in 2015, with next-generation therapeutic antibody revenues reaching $2.3 billion in 2015 according to Visiongain reports published in September and November 2011 (2, 3). Biosimilar antibodies will also begin to enter established markets as regulatory authorities clear approval pathways for them. Most…
NIR Spectroscopy for Process Monitoring and Control in Mammalian Cell Cultivation
The quality by design (QbD) and process analytical technology (PAT) approaches have shown significant benefit in the classical pharmaceutical industry and are now strongly influencing bioprocessing. Monitoring critical process parameters (CPPs) during biotechnological cell cultivations is essential to maintaining high efficiencies and quality. Commercial sensor systems for real-time inline monitoring are available for some parameters, such as pH or the concentration of dissolved oxygen (DO). For others such as glucose concentration, total cell count (TCC), and viability no robust online…