For HIC separations, parameters other than resin surface modifications can be employed to enhance performance. This application note addresses the electrolyte composition of the mobile phase as one parameter responsible for protein adsorption and desorption. The results presented illustrate the benefits regarding capacity and selectivity in HIC of often neglected salts and their mixtures.
Downstream Processing
Analysis By Size and Charge
An early BPI Lab article addressed the power of liquid chromatographic separations for biopharmaceutical laboratory use (1). Such techniques separate biomolecules based on a number of different properties: size, solubility, hydrophobicity/-philicity, binding affinity. The next most powerful means of separation — and thus high-resolution identification — of nucleic acids and proteins/peptides is based primarily on electrostatic properties: electrophoresis. Although it doesn’t really work in a process or preparative setting, it is a fundamental technique in modern biopharmaceutical laboratories, where it…
Accounting for the Donnan Effect in Diafiltration Optimization for High-Concentration UFDF Applications
The biopharmaceutical industry is targeting high-concentration protein formulations to enable subcutaneous administrations. Such administration can provide better patient convenience than intravenous administration. One challenge associated with high-concentration formulations is increased electrostatic interaction between proteins and excipients. That is a result of increased protein-charge density at high protein concentrations. Such interactions can create an offset between excipient levels in final products and diafiltration buffers in ultrafiltration processes. The effect of such electrostatic interactions in a membrane process is known as the…
Separation of Monoclonal Antibody (mAb) Monomer from its Half-body using Size Exclusion Chromatography
Recent research has shown an interest in mAb half-bodies as therapeutic vectors as they can be further targeted for conjugation, enzyme labeling, or antibody immobilization. The TSKgel SuperSW mAb HR is able to achieve high resolution between the mAb monomer, the mAb half-body, and fragments due to its unique pore-controlled technology optimized for mAb analysis, as well as its smaller 4 ÎĽm particle size.
Monoclonal Antibody Purification with a High Capacity Protein A Resin
“Protein A resins constitute a substantial cost in state-of-the-art mAb purification processes. Factors such as operating cycles, capacity, and mAb titer can have an impact on total costs associated with mAb purification. The purification of a monoclonal antibody from crude feed stock using TOYOPEARL® AF-rProtein A HC-650F, a high capacity protein A resin from Tosoh Bioscience, show that this particular high capacity protein A resin delivers highly pure antibodies at yields approaching 90%.”
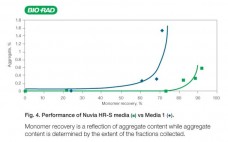
Introducing Nuvia HR-S Chromatography Media
Nuvia HR-S media is a new strong cation exchanger that has been optimized for particle size and chemistry that provides exceptional resolution and high recovery. Nuvia HR-S media demonstrates fast mass transfer kinetics, excellent flow characteristics, and robust chemical stability against common caustic cleaning protocols. Its excellent scalability gives process developers the confidence that results obtained on the bench will be reproducible for large-scale downstream manufacturing. Nuvia HR-S media is the preferred solution for intermediate and final polish applications where…
TOYOPEARL AF-rProtein A HC-650F Host Cell Protein Removal
The following paper compares the host cell protein (HCP) removal capabilities of TOYOPEARL AF-rProtein A HC-650F, TOYOPEARL AF-rProtein A-650F, and another commercially available high capacity protein A resin.
Process Improvements Increase Production Capacity of a Legacy Product
Implementation of postlicensure process improvements in the biopharmaceutical industry can benefit patients and drug manufacturers alike. Here we demonstrate through a case study how a change to the cell culture medium and process can be taken from proof of concept through scale-up to demonstration of feasibility. We further illustrate the scope and complexity of implementing a change in commercial manufacturing to realize significant benefits such as increased production capacity over an existing legacy process. The Importance of Postapproval Improvements Drug…
Impact of Process Interruption on Virus Retention of Small-Virus Filters
Manufacturers of biopharmaceuticals using mammalian cell culture must have processes in place to minimize the likelihood of virus contamination of their products. Regulatory agencies provide guidelines for testing strategies and best practices to assure raw-material safety and control of the manufacturing process. Safety assurance relies on an interdependent matrix of managed risks, including characterization and control of raw materials, extensive testing of process intermediates, and demonstration of the virus removal capabilities of purification unit operations Figure 1: () A dedicated…
Effects of Pressure Sensor Calibration Offset on Filter Integrity Test Values
Food and Drug Administration (FDA) and European good manufacturing practices (GMPs) require integrity testing of sterilizing-grade filters for producing injectables and other biologics. The diffusion test (also called the forward-flow test) and bubble-point test (also called the disk test) of a sterilizing-grade filter are both filter-integrity tests. The accuracy of both relies on calibration of a pressure sensor in the respective integrity test unit. Calibration of the pressure sensor of a filter-integrity testing device is an essential part of quality…