A number of regulatory guidelines recommend preuse integrity testing of critical sterilizing liquid filters for aseptic processing (1–3). Before sterilization, a preuse test will confirm that a filter is installed properly and was not damaged during shipment or handling. Performing a preuse test after sterilization detects damage that may have occurred during the sterilization cycle. Testing after sterilization limits risk, so it is a practice applied based on risk assessment. Because it is perceived to reduce business loss risk, preuse…
Downstream Processing
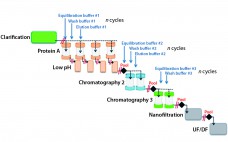
Accelerated, Seamless Antibody Purification: Process Intensification with Continuous Disposable Technology
Process intensification through continuous manufacturing has been practiced in the chemical, petrochemical, and food industries for years and has gained much interest among biopharmaceutical manufacturers (1). Key drivers encouraging biomanufacturers of therapeutic molecules to convert batch processes into continuous operation include flexibility, productivity, cost effectiveness, and product consistency. Continuous upstream processing has been demonstrated for the manufacture of a broad range of molecules, including complex/labile proteins such as enzymes (2) and monoclonal antibodies (3). Recent publications have reported successful application…
Prepacked Chromatography Columns: Evaluation for Use in Pilot and Large-Scale Bioprocessing
Time to market, resource requirements, cost, and flexibility are key considerations in designing purification processes suitable for manufacturing biopharmaceutical products. Over the past decade, many advances have been achieved in disposable processing systems that have allowed for increased processing at a lower cost. That is in part attributable to reductions in necessary resources, changeover costs, and cleaning-validation requirements. Large-scale, prepacked chromatography columns have recently become available for clinical and commercial manufacturing, and they represent a growing trend in the industry.…
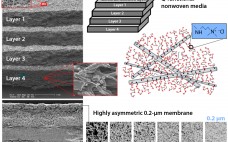
Virus-Filtration Process Development Optimization: The Key to a More Efficient and Cost-Effective Step
Size-exclusion–based parvovirus filtration is an important step toward drug product safety in biopharmaceutical production. However, once a virus filter is in place, and the required virus safety is ensured, less attention typically is paid to its optimization within the process. That might seem odd given that virus filtration can be one of the more expensive downstream processing steps ($/g protein processed). Most likely, the lack of attention can be attributed to aggressive timelines, limited process development resources, and the virus…
Automated Purification of Native and Recombinant Proteins Using Multidimensional Chromatography
In traditional sequential chromatography, columns are run as separate entities. The process requires significant hands-on time and constant manual intervention. By contrast, automated chromatography technology provides the same results more efficiently and reliably and frees researchers to focus on other tasks, thereby shortening protein purification times from days to hours. For drug discovery, purifying protein samples is required to generate enough materials for research experiments. But the process is complex and time consuming. It involves repeated single-column purifications, careful analysis,…
The Secret Life of Protein A
Affinity chromatography with protein A has become the foundation for purification of nearly every therapeutic IgG in commercial production. One of the features most responsible for its success has been its compelling simplicity. IgG binds. Contaminants do not. Load, wash, and elute pure IgG. In the real world, however, protein A does not elute pure IgG. It typically contains several hundred to a few thousand parts per million (ppm) contamination by host-cell proteins (HCPs) and other contaminants. Numerous studies demonstrate…
Characterization of Subvisible Particles in Vaccine Development
Vaccine manufacturers want meaningful information that can be linked to process knowledge to increase product quality and eliminate patient safety issues. New requirements from the United States Pharmacopeial Convention (USP) and the U.S. Food and Drug Administration (FDA) put pressure for root cause analysis to understand impact on changes during process and product development. The new demands involve not only size and distribution but deeper characterization that involves morphology and composition studies as part of particle characterization. Nanoparticles like virus,…
Optimization and Scale-Up of HCIC-Based MAb Purification Processes, Part 2
In multistep schemes, hydrophobic charge-induction chromatography (HCIC) has been shown to contribute effectively to clearance of Chinese hamster ovary (CHO) host-cell proteins (CHOPs), DNA, and viruses. When used for capture chromatography, HCIC can provide better aggregate clearance than protein A sorbents can. Chen et al. enhanced clearance of aggregates, CHOPs, and product- related impurities by controlling HCIC based on both pH and the presence of binding-promoting salt in the wash and elution buffers used (1). Taken together with our findings…
Anion-Exchange Chromatographic Clarification: Bringing Simplification, Robustness, and Savings to MAb Purification
Monoclonal antibodies (MAbs) are the most prominent and successful therapeutic proteins in the pharmaceutical industry. More than 35 MAbs have been approved to treat a range of conditions, and hundreds more are in development (1, 2). Once, the upstream cell culture process was considered the bottleneck to producing high antibody doses required for treatment, but recent advances in cell culture technology have boosted antibody titers to the range of 5–10 g/L (3). That increase in productivity has shifted focus onto…
An Industrial Platform Solution for Antibody Fragment Purification
Compared with traditional approaches such as chemotherapy and radiotherapy, monoclonal antibodies (MAbs) have become the most successful cancer treatments in the past 20 years (1). With great clinical success in many therapeutic areas, MAbs now account for >40% of the entire biotechnology drug market, and sales are projected to be >US$160 billion over the next few years in the United States alone (2). More than 35 MAbs have been approved for clinical use, and hundreds more are filling industry development…