Continuous and semicontinuous manufacturing systems have been used for many years in numerous sectors — including the automotive, food and beverage, oil refining, chemicals, pulp and paper, electronics, metal smelting, steel making, and waste-water treatment industries. Most of these industries are capital intensive and switched to flow manufacturing to increase productivity and flexibility; reduce cycle times, inventory, waste, and costs; and achieve enhanced product quality. Despite the capital intensive nature of drug manufacturing, the biopharmaceutical industry has lagged behind these…
Business
A Turning Point for US Biosimilars
The next 12–18 months could be a critical time for biosimilars in the United States (1). This product class has grown rapidly since passage of the Biosimilars Price Competition and Innovation Act (BPCIA) of 2010. That landmark legislation allowed for biosimilar market approvals based on previously approved “reference products,” creating an expedited pathway that reduces biosimilar development costs and speeds regulatory review so patients get faster access to essential medicines. Increased competition from biosimilars could save the US healthcare system…
Automation in Cell Therapy Manufacturing
The concept of automation conjures up images of robots on assembly lines or perhaps automobiles replacing horse-drawn carriages. In both examples, automation provides an ability to work tirelessly, with reproducible high-quality outputs at increased speed. For cell therapy, automation can be used to increase the scale of cell culture operations (e.g., bioreactors replacing flasks) and allow the use of closed systems that can protect cell products from contamination with adventitious agents from the environment or operators themselves. Closed systems also…
Collaboration Will Drive Regenerative Medicine: Toronto Development Center Will Help to Advance the Field
With support from the federal government of Canada, GE Healthcare and the Centre for Commercialization of Regenerative Medicine (CCRM) are pushing into new frontiers to advance the progress of cell therapy and regenerative medicine. When I first met Michael May, president and chief executive officer (CEO) of CCRM, both our organizations had been exploring opportunities in parallel to drive the cell therapy industry forward. CCRM’s mission is to create and sustain a global nexus for company creation, technology and cell…
Achieving Competitive Advantage in the Biopharmaceutical Industry
Drug development is a complex process that is associated with high drug-candidate attrition rates, long development times, and high costs (1, 2). Drug development costs have increased over the past two decades, with current average development cost of about US$2.6 billion, of which $1.4 billion is the direct cost (1, 2). On average, drug development takes at least 10 years to market authorization (2). Biopharmaceutical companies often follow a strategy of developing drugs for multiple clinical indications and biological targets…
CMC Strategy Forum Special Focus Series: Part 2 Product-Related Impurities, An Overview
Introduction by Cheryl Scott The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of forum meetings are published in BioProcess International and on the CASSS website. This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment…
The Year of Data Integrity: 2015 Brought a Worldwide Focus on Training, System Design and Control, and Data Management
Each year, regulatory agencies from around the world focus on critical aspects of the pharmaceutical quality management system, bringing awareness to the industry and continuing to effect positive change. In the past five years, risk assessments, electronic records, and outsourced activities have been in the spotlight. As 2015 closed out, it was clearly the year of data integrity. In March 2015, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) published its GMP Data Integrity Definitions and Guidance for Industry,…
Clinical Supply Chain: A Four-Dimensional Mission
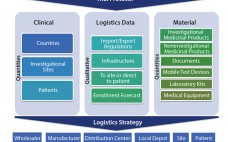
A clinical supply chain fulfills perfectly all four characteristics of what Packowski describes as a “VUCA” (volatility, uncertainty, complexity, and ambiguity) world (1). In commercial markets, supply chains depend predominantly on consumer orders. For global drug development programs, both investigators and patients can be considered end consumers. The international journey of a specific investigational medicinal product (IMP) includes all of the following: global sourcing of comparators, manufacturing, storage, distribution, site/patient (consumer) management, and return and destruction of the IMP. Application…
Opportunities in Latin America Beyond the Olympics
Olympics fans aren’t the only ones turning their eyes toward Brazil. According to JLL’s fourth annual Life Sciences Outlook, the 2016 Summer Games host nation is also one of the world’s top 10 “Global Clusters to Watch,” thanks to its proven capacity for medical device manufacturing and a growing healthcare market. Increasingly, Latin America overall has been on the radar for life sciences companies seeking greater operational efficiency and opportunity, expanded market access, promising demographics, lower-cost land and labor, and…
Responding to an FDA Form 483: A Five-Step Approach
When the US Food and Drug Administration (FDA) inspects your company’s biomanufacturing facility, investigators use the FDA Form 483 to record observations and findings (1). Such inspections typically review all good manufacturing practices and good laboratory practices (GxP) quality systems documents. If those investigators find compliance issues, they deliver a summary of their observations and findings using a Form 483, a copy of which will be provided to your company at the end of the inspection visit. How to Respond…