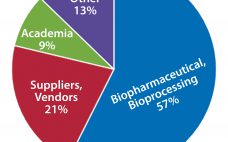
Assessing the need for training that addresses the varied needs of your employees requires looking far beyond a one-size-fits-all approach. Few companies, however, have the internal resources to develop truly comprehensive programs on their own. Successful training approaches are those that enable end-users, organizations, and suppliers to share needed equipment and expertise. At the 2016 BioProcess Theater at Interphex, Gary Gilleskie (BTEC’s director of operations) and Scott Sommer (a technical fellow at Renmatix) detailed examples of successful training programs for…
Business
Measuring the Impact of Investments in Professional Development: A Virtual Roundtable
Well-designed education, training, and professional development programs and partnerships add considerable return on investment to stakeholders across the biopharmaceutical manufacturing industry. As biomanufacturers, suppliers, and regulatory bodies share similar workforce needs, focused education and training efforts can lead to increases in productivity, yield, and employee engagement and retention. In addition, education and training programs can decrease the incidence of deviations, which are costly to investigate and remediate. Yet when compared with other business needs, training, development, and human performance initiatives…
Exploring Academic Models for Biomanufacturing Education
Undergraduate and/or graduate programs in physical and life sciences can provide a solid background for science and engineering students who are interested in careers in biotechnology research and development. Yet many such programs have not adequately prepared students for careers within and related to the biopharmaceutical industry. In today’s globally competitive job market, developing a workforce pipeline for the bioprocess industry requires academic programs that equip students with knowledge, skills, and theory surrounding the equipment, methodologies, processes, and regulatory requirements…
Demographics and Trends: Results from a Joint BPI/BTEC Survey on Training
Training is an investment. And it’s one that the authors, contributors, and other individuals behind this supplement understand well. However, the biopharmaceutical industry is much larger than these few individuals. It’s just as important to understand the motivators, drivers, and training-related challenges faced by the industry at large. So BPI and BTEC jointly administered a short survey to gather some basic information from the magazine’s readership about the value and role that training plays in their organizations. According to survey…
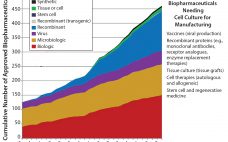
BioPhorum Operations Group Technology Roadmap, Part 1: Four Trends Shaping the Future of the Industry
What prompts over 100 biopharmaceutical manufacturers, leading academics, supply partner R&D heads, regulators, and worldwide regional hubs to get involved in a major project? It’s when that project identifies the future technology needs of the biopharmaceutical manufacturing industry and accelerates its collective innovation. In February 2015, the BioPhorum Operations Group (BPOG) Technology Roadmapping steering committee met in Washington DC to create the first “technology roadmap” for the biopharmaceutical manufacturing industry. The biopharmaceutical market has been experiencing dramatic changes: explosive growth…
India’s Next Steps: Quality Improvements Target International Markets
India’s position as a global participant in small-molecule generic drugs, vaccines, and enzymes has been proven over decades. The country is one of the most populous and fastest-growing regions in the world, both economically and technically. But India’s potential as a biologics participant has not been realized. Its competence as a global biologics producer has not yet caught up. Global industry concerns regarding the country’s position in the (bio) pharmaceutical industry haven’t changed much over the past eight years since…
Funding for Life-Science Ventures: Accelerating Innovation in Tools and Services
As a cofounder of Wave Biotech (now a division of GE Healthcare), my partners and I often struggled with critical choices regarding partnering and funding opportunities. Every new, attractive, and potentially disruptive technology will court attention once it experiences some modest adoption and acceptance, even while attempting to “fly under the radar” of major players. The challenge for life-science entrepreneurs is how best to navigate those decisions and select the right path as company founders. Weighing and evaluating potential partners…
Postapproval Changes for Biopharmaceutical Drug-Substance and Drug-Product Manufacture: Regulatory Complexity and Impact
Pharmaceutical products save or improve the lives of millions of people each year. Thorough regulatory review of chemistry, manufacturing, and controls (CMC) information is critical to ensure drug product safety, quality, and efficacy as well as to secure patients’ continuous access to such products. But achieving all of that at an effective cost is difficult. Companies race to launch products to patients as soon as possible after clinical efficacy is demonstrated. Biomanufacturers often need to make changes such as increasing…
Collaboration Is Key to Innovation in Biotechnology
A new report from Thomson Reuters shows that innovation in biotechnology declined slightly in 2015, and biotech is the only one of a dozen worldwide industries examined to show that kind of decline (1). To measure innovation, compilers used metrics such as patents filed and scientific literature cited. Looking at the details, however, the dip was just a 2% drop from 42,584 events in 2014 to 41,624 in 2015. The same dynamics had revealed a 7% increase in innovation from…
Addressing the Challenges of Developing Biopharmaceutical Drugs
The biopharmaceutical industry is enjoying considerable success. Its products account for about a fifth of world pharmaceutical revenues, which are growing at twice the pace of those generated by most traditional chemically synthesized drugs. Biopharmaceuticals populate the list of best-selling drugs, and a number have achieved blockbuster status. Biotechnology stocks have outperformed the general market as investment has flowed into the industry. As with other highly profitable markets, the market for biopharmaceuticals has become increasingly competitive. Reflecting this fact, in…