The biopharmaceutical industry continues to invest heavily in technologies for identification of predictive biomarkers. Drug developers want not only to find quantitative evidence that their therapies will work as designed, but also to anticipate which patient populations will respond positively to those regimens. Doing that could streamline clinical trials, accelerate approvals, and ultimately improve patient outcomes. Advances in next-generation sequencing and increases in computational capability now are facilitating biomarker inquiries, especially in the realm of immunooncology. However, predictive biomarkers remain…
Analytical
Making Safe and Effective CAR T Cells: How Droplet Digital PCR Can Improve Their Quality Control
Chimeric antigen receptor (CAR) T cells first entered US clinics in 2017 (1), and this therapeutic modality holds tremendous potential as one of the most effective forms of personalized cancer care ever to reach patients. The revolutionary impact of CAR T-cell therapy comes from its ability to rewire our own immune defenses to kill cancer cells: It essentially modifies a patient’s naturally existing immune cells to boost their recognition and attack of cancer cells so that the person’s own immune system…
Analytical Support for Biologics: A Conversation with Almac Sciences
Almac Sciences (a member of the Almac Group) recently expanded its suite of analytical solutions to include biologics testing. This follows a 2019 expansion of the company’s facility in Athlone, Ireland, where it provides a comprehensive range of flexible pharmaceutical testing services to support drug development programs adhering to industry regulations and good manufacturing practice (GMP) standards. “Biologics have gained huge traction in the past decade and are poised for stronger growth in the coming years with potential to significantly…
Ultrasonic Flow and Bubble Sensors: Optimize Process Quality in Single-Use Bioprocessing Applications
Process monitoring in laboratory and production environments enables continuous control and optimization of critical process parameters. Hence, the early detection of errors is an effective means of increasing process efficiency and reproducibility, improving quality and safety parameters, and reducing long-term costs. Highly precise and flexible noncontact clamp-on flow and bubble sensors are useful instruments to fulfill these goals. They can be applied effectively to buffer and media preparation, chromatography and filtration systems, bioreactors and fermentors, feed and transfer lines, and…
Ultracentrifugation for Recombinant Adenoassociated Virus Therapies
Quantitation of different capsid species in viral-vector gene-delivery drugs, including recombinant adenoassociated virus (AAV) therapies, is essential for proper assessment of critical quality attributes before regulatory approval and during commercial production. If rAAV full-capsid percentages are maximized, and variants such as empty or partially-filled capsids are reduced as much as possible, then potency is increased for improved dosing and tolerance outcomes. Traditionally, characterization of AAV purity with respect to empty, full, and other variants has been performed by techniques such…
Quality Risk Management for Filter Integrity Testing: Compliance with EMA’s Future Annex 1
Quality risk management (QRM) is a systematic process for assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. Although QRM is not new (1), the regulatory focus on QRM will increase with the arrival of the European Medicines Agency’s (EMA’s) Annex 1 (2), which was reviewed by the US Food and Drug Administration (FDA), the World Health Organization (WHO), and the Pharmaceutical Inspection Convention Scheme (PIC/S). Integrity testing of sterilizing-grade filters is…
Endotoxin Questions and Answers
Endotoxin — also known as lipopolysaccharide (LPS) — is a large molecule made up of a lipid and a polysaccharide portion found in the outer membrane of each Gram-negative bacterial cell. Examination of endotoxins is necessary for microbial and animal cell-culture operations that produce active pharmaceutical ingredients (APIs) and vaccines. If a cell culture is contaminated with endotoxins, it could affect cell growth and function (thus potentially affecting product quality). Biopharmaceutical companies need to understand the endotoxin levels within their…
Ask the Expert: Mass Spectrometry for Relative and Absolute Quantitation of Process-Related Impurities
Qualitative and quantitative analyses of process-related impurities are critical to manufacturing a safe, high-quality drug product. During his 17 June 2020 “Ask the Expert” webcast, Steven Broome (BioPharmaSpec) described using mass spectrometry (MS) for such analyses. Focusing on isopropyl-β-D-thiogalactopyranoside (IPTG), kanamycin, and host-cell proteins (HCPs), Broome overviewed best practices for using MS to detect and quantitate low levels of impurities. Broome’s Presentation Chemicals used in biotherapeutic production can be present as impurities in the resulting drug product. These impurities can…
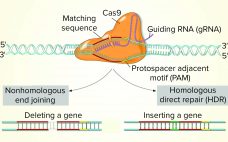
Use of CRISPR and Other Gene-Editing Tools in Cell Line Development and Engineering
While the role of biologics in treating human diseases has evolved dramatically over the past decade, so has genetic engineering. Rational genetic engineering to enhance biotherapeutic proteins has become a reality catalyzed by publication of the genome sequences of multiple Chinese hamster ovary (CHO) cell lines. Novel “designer” CHO cells modulate posttranslational modifications (PTMs) of recombinant proteins by genome editing, and it is now possible to knock-in or knock-out genes of yeast and mammalian cells precisely (within one DNA base…
Development of Patient-Focused Commercial Specifications: Understanding Clinical Relevance and Criticality of Quality Attributes
The CASSS chemistry, manufacturing, and controls (CMC) Strategy Forum on 23 January 2019 in Washington, DC, was entitled, “The Development of Patient-Focused Commercial Specifications Through Understanding of Clinical Relevance and Criticality of Quality Attributes.” This forum covered the definition, identification, control, and management of patient-focused attributes throughout the life cycle (from discovery through approval) of biological products, including vaccines. Participants investigated how to differentiate through the product development life cycle which attributes are “clinically meaningful” from those applied for manufacturing…