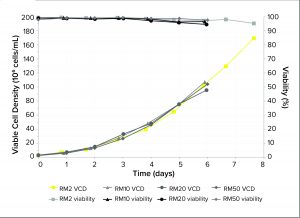
Figure 1: Viable cell density (VCD) and viability of Chinese hamster ovary (CHO) cells cultured in RM perfusion bioreactors with working volumes from 2 L to 50 L
The high costs of and limits on global accessibility of biologics such as monoclonal antibodies (MAbs) are focusing the biopharmaceutical industry’s attention on strategies for rapid, economical development of such therapies. Process intensification is one approach to help shorten manufacturing timelines and reduce cost of goods (CoG) (1, 2). Today, process intensification in upstream cell culture enables biologics manufacturing in facilities with smaller footprints and lower scale-up volumes than was possible before. Intensified processing of Chinese hamster ovary (CHO) clones that produce MAbs is being developed in the seed train of upstream cell cultures (3) to support generation of high–cell-density (HCD) cell banks and processes to reduce plant size, capital investment, and overhead costs while increasing productivity.
In traditional practice, MAb manufacturing relies on seed ratios of ~1:5 (3) with an initial inoculum of 0.3 × 106 cells/mL (4) using a seed train of five to eight transfer steps. Standard seed trains typically begin with shake flasks in the postthaw expansion (N – 8 to N – 5) and then move through a rocking-motion (RM) 5–50-L bioreactor (N – 4 to N – 3) to seed a series of N – 2 to N – 1 stirred-tank (STR) bioreactors (200 L and 500 L) ending in a production culture (N) with a volume of 2,000 L. Adopting an intensified cell culture approach at N – 4 to N – 1 by, for example, using 2D RM bioreactors in perfusion mode (where cells stay in the exponential growth phase throughout the entire culture) could increase viable cell densities to 100 × 106 cells/mL and drive seed ratios of >1:100. That allows skipping of process-transfer steps. For example, HCD cell banks can be thawed directly and inoculated into RM bioreactors at higher volumes (0.5–2 L depending on the HCD cell-bank volume), thus bypassing cell-culture expansion in shake flasks and the potential errors and contamination issues associated with manual handling of flasks. Also, with perfusion in the N – 1 seed train, cells can reach higher densities for inoculating a pilot or production bioreactor directly without process transfer through multiple intermediate-scale STR bioreactors. That reduces both the number of steps and the total number of seed-train days, thus reducing initial investment and operational CoG. However, not all CHO clones that produce MAbs can be cultured in intensified processing because high cell densities can compromise protein expression levels and genetic stability (5).
Our goal was to explore the possibility of culturing a CHO clone that expresses a commercial MAb therapeutic in HCD for an intensified, scalable cell-culture seed-train protocol. We investigated whether single-use RM perfusion bioreactors (working volume 1–25 L) could achieve a reproducible cell culture with at least 100 × 106/mL viable cell density (VCD) at different working volumes. The HCD culture was monitored using process analytical technology (PAT) at all scales. When the culture in the RM bioreactor (1-L working volume) reached the required HCD, a culture volume was used to seed a pilot-scale STR single-use bioreactor (200 L) automatically, which then ran in fed-batch mode. During the pilot run, we measured cell density and viability along with the MAb’s critical quality attributes (CQAs), then compared those results with the same MAb produced through a standard seed train. The results established whether a HCD perfusion seed train can inoculate a standard manufacturing N-stage bioreactor to reduce the number of transfer steps and whether it is possible to use a PAT-based control regime for automated inoculation of N-stage processes to reduce inoculation volumes.
Materials and Methods
Cell Line: We chose a CellcaCHO-DG44 cell line expressing a commercially available human MAb to investigate the practicality of using HCD perfusion culture to inoculate a pilot-scale bioreactor. The cell line, with its associated media and cell-culture parameters, was selected for proof-of-concept studies because the fed-batch process used to produce this MAb has been well-characterized by Sartorius.
Bioreactors: The Biostat RM 20/50 bioreactor (Sartorius) was chosen for seed-train studies because it continuously renews the cell-culture medium, enabling mass transfer between the headspace and medium for efficient, low-shear mixing. The system was used with single-use Flexsafe RM perfusion bags (1–25-L working volume) constructed of pharmaceutical grade low-density polyethylene, which is used throughout the entire range of RM and STR bioreactor bags to ensure consistent quality. The bags each feature an integrated perfusion membrane at the bottom, forming a compartment for removing cell-free media during the perfusion process for minimal loss of or damage to the cells. The RM perfusion bioreactors scale from 2 L to 50 L (working volume 1 L–25 L) using a common RM bioreactor design to simplify process transfer.
A Biostat STR bioreactor (200 L) with single-use Flexsafe STR bags was used as the pilot-scale single-use bioreactor. The Sartorius STR bioreactor range scales from 50 L to 2,000 L and uses the same established bioreactor design principles as stainless-steel bioreactors. All bags have similar geometries across scales (5) to facilitate process transfer and scale-up (4).
Proof-of-Concept Studies
Seed Train Study: To demonstrate the performance and scalability of cultures in RM perfusion bioreactor bags, we cultured CHO cells for six days in perfusion bioreactor bags of 2 L, 10 L, 20 L, and 50 L (working volumes of 1 L, 5 L, 10 L, and 25 L) using proprietary media and feeds at a minimum cell-specific perfusion rate (CSPR) of 50 PI (cells/day). The cells were cultured at 36.8 °C, pH 6.95, and DO 60% (maintained by increasing the rocking rate at a 10° angle). On-line PAT sensors, including the BioPAT ViaMass sensor, were used to monitor and control the culture’s VCD and viability and enable automatic feeding. The 2-L culture was cultivated for an additional two days under perfusion conditions to determine a maximum VCD.
Pilot Bioreactor Studies: A VCD model from the RM perfusion bioreactor was created from the signal from the BioPAT ViaMass sensor. With this model, the VCD was measured on-line constantly, and when cells reached a trigger point of 100 × 106 cells/mL, a cell inoculum volume calculated to produce an initial cell density of 0.33 ± 0.04 × 106 cells/mL was transferred automatically from the RM perfusion bioreactor to the pilot scale STR bioreactor. Cells cultured in the perfusion bioreactor (400 mL at approximately 100 × 106 cells/mL) were used to seed a Biostat STR 200-L single-use bioreactor. Cells were cultured for 12 days using proprietary media and feeds at 36.8 °C, pH 7.1, and DO 60%. Feeds were added after a three-day batch phase on day 4. VCD and cell viability data were measured and collected by the BioPAT ViaMass sensor. A cell free supernatant was sampled for N-glycan profiling to determine MAb product quality and titer. The results were compared with historical data from a standard seed train for which cells were cultured in standard Biostat RM 20/50 bags (14 L at approximately 3 × 106 cells/mL) and used to inoculate a Biostat STR 200 L single-use bioreactor.
Results and Discussion
Performance of Seed Train: The results (Figure 1) show that in six days, the RM perfusion bioreactors can grow CHO cell cultures with >90% viability and with high cell densities of >100 × 106 cells/mL. That represents a 33-fold increase in VCD compared with an inoculum from a standard RM bioreactor. Over eight days, in a 2-L bag, these perfusion bioreactors support cell culture to VCDs of 170 × 106 cells/mL with >90% viability, demonstrating that cellular oxygen demand can be supported at such high cell densities.
Our VCD and viability results are consistent at all scales tested up to 50Â L. The reproducibility and scalability of HCD cultures using the perfusion bioreactors shows that this method can produce cells with high enough VCDs to seed 200-L pilot bioreactors directly, indicating the potential for inoculating 2,000-L production bioreactors using a suitable HCD culture, e.g., by using a RM 10 or 20.

Figure 2: Comparison of VCD and viability of CHO cells in 200-L pilot-scale fed-batch culture inoculated with cells from standard or intensified seed trains
Pilot-Scale Study: Cells in proprietary media from the RM perfusion bioreactor were used to seed a 200-L pilot-scale bioreactor using an automated PAT sensor-controlled inoculation set with a target initial cell density of 0.33 ± 0.04 × 106 cells/mL. That resulted in an actual starting cell density of 0.37 × 106 cells/mL, demonstrating that the PAT sensor can achieve inoculation precision comparable with manual seeding. In total, 0.4 L of culture from the intensified seed train was used to inoculate the pilot-scale bioreactor compared with needing over 13 L of cell culture from a standard seed train. Thus, using HCD cells to seed the pilot-scale bioreactor required over 30-fold less inoculation volume than when using cells from a standard seed train.
Fed-batch results (Figure 2) from the pilot-scale bioreactor seeded with HCD cells from the RM perfusion bioreactor and the traditional fed-batch cell culture show comparable growth characteristics over the entire process run. Both achieve a maximum VCD of 25 × 106 and viability of >90% in seven days. The MAb titers also were similar, with 3.5 g/L achieved using the standard seed train compared with 3.2 g/L from the intensified seed train. Those results indicate that using HCD cells to seed pilot-scale bioreactors has no adverse effect on cell growth, viability, or titer over a standard 12-day fed-batch run at 200-L scale in a single-use STR bioreactor.
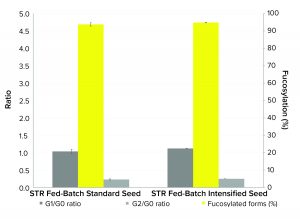
Figure 3: Comparison of MAb critical quality attributes (CQAs) expressed by Chinese hamster ovary (CHO) cells in 200-L pilot-scale fed-batch culture inoculated with cells from standard or intensified seed trains
Product Quality: CQAs of the MAb product from fed-batch cultures inoculated with standard and from intensified seed trains (Figure 3) showed similar trends in glycan profiles of G2/G0 and G1/G0 ratios and with 95% fucosylated forms produced. The performance results of the cells cultured in the pilot-scale bioreactor from the intensified seed train shows that using HCD cells to seed a pilot-scale bioreactor does not affect product quality. That indicates the usefulness of this approach for a hybrid perfusion/fed-batch process scale-up.
Productivity and Quality
The proof-of-concept studies presented here show that single-use RM perfusion bioreactors can achieve HCD cell cultures (>100 × 106 cells/mL) with >90% viability at every scale from 1 L to 25 L working volumes. The studies also demonstrate that HCD cells (inoculated by means of automated process control) and cells from a standard fed-batch culture used to seed a 200-L STR bioreactor produce comparable cell growth, viability, titer, and MAb CQA profiles. Our results indicate that using this intensified seed-train approach can increase productivity without compromising product quality and could potentially increase the cost-effectiveness of biologics production.
References
1 Anderlei T, et al. Facility of the Future. DECHEMA Report 2018; https://dechema.de/dechema_media/Downloads/Positionspapiere/SingleUse_FoF+2018+engl.pdf.
2 BioPhorum Operations Group Report. Biomanufacturing Technology Roadmap 2017: Process Technologies; https://www.biophorum.com/download/process-technologies.
3 Wittmann C, et al. 2016 Industrial Biotechnology: Products and Processes. Wiley VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; https://doi.org/10.1002/9783527807833.
4 Matuszczyk J-C, et al. A Rapid, Low-Risk Approach for Process Transfer of Biologics from Development to Manufacturing Scale. BioProcess Int. 18 (5) (2020): 44–51; http://lne.e92.mwp.accessdomain.com/sponsored-content/biostat-str-bioreactors-a-rapid-low-risk-approach-process-transfer-of-biologics-from-development-to-manufacturing-scale.
5 Rees-Manley A. Evaluation of a Small-Scale Perfusion Mimic for Intensified Processes. Gen. Eng. News 2019; https://www.genengnews.com/resources/tutorial/evaluation-of-a-small-scale-perfusion-mimic-for-intensified-processes.
6 Noack U, et al. Single-Use Stirred Tank Reactor BIOSTAT CultiBag STR: Characterization and Applications. Single-Use Technology in Biopharmaceutical Manufacture. Eibl R, Eibl D (Eds). Wiley: Hoboken, NJ 2010: 225–240; https://doi.org/10.1002/9780470909997.ch19.
Corresponding author Jens-Christoph Matuszczyk is manager, bioprocessing upstream, Sartorius, Göttingen, Germany; 49-551-308-4245; Jens.Matuszczyk@sartorius.com. Johannes Lemke is a scientist and Markus Schulze and Sabrina Janoschek are PhD students. Gerben Zijlstra is a global technology consultant, and Gerhard Greller is a senior scientist at Sartorius Stedim Biotech GmbH. Editorial contact: Dominic Grone, Sartorius; 49-551-308-3324; dominic.grone@sartorius.com.
