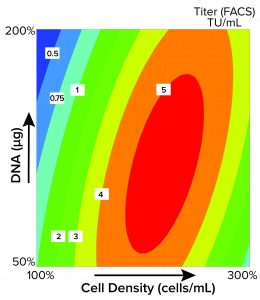
Figure 1: Contour plot obtained using MODDE software shows the optimal seeding density for viral vector production. The quantity of plasmid DNA (µg) is plotted against cell density (cells/mL) with increasing infectious titer over that of the initial process as the response, in this case, increasing from 0.5 (blue) to 5 (red); FACS = fluorescence-activated cell sorting, TU = transduction units.
With recent developments and successes in cell and gene therapy, the biopharmaceutical industry is facing increased demand for safe and efficient delivery systems (1). Viral vectors, including adenoviruses (AV), adenoassociated viruses (AAV), and lentiviruses (LV), are among the most common delivery agents because they infect mammalian cells efficiently. Suspension cultures have become a popular choice for robust and scalable viral manufacturing systems. Using stable cell lines that integrate all or part of the viral production elements adds further benefits by improving consistency and reducing manufacturing costs. Rapid process optimization is key to increasing a platform’s efficiency and reducing the time to reach the market.
In this study, we sought to develop an optimized protocol for reliable, high-titer production of LV vectors using OXGENE’s XLenti prepackaging suspension cell line with single-plasmid transfection. The XLenti cell lines are designed for high performance and easy adoption in manufacturing processes and are the first to be developed using a completely animal-component–free process.
To optimize LV production in the XLenti prepackaging cell line, we used a design of experiments (DoE) approach using MODDE software (from the Umetrics data analytics suite) in combination with an automated high-throughput microscale bioreactor system, the Ambr 15 bioreactor. As anticipated, this platform accelerated viral production process optimization by allowing us to screen multiple process parameters simultaneously, leading to significant improvements in viral production yield from our system.
Materials and Methods
Cell Culture: Prewarmed production media (the BalanCD HEK293 cell line (Irvine Scientific) with an Ultraglutamine 1 supplement (Lonza (BE17-605E/U1) was added to the Ambr 15 vessel to fill 80% of its volume. The XLenti prepackaging cell suspension was added at 10% v/v of working volume to achieve the target cell density, which is product- and cell-line dependent. Cells were maintained in the bioreactor at 37 °C and pH 6.8–7.4, with 40% dissolved oxygen (DO) and a high stirring speed.
Transfection: To produce LV vectors from the XLenti cell line, we transfected the cells with a single plasmid. That mix was prepared by combining the plasmid and transfection reagent (PEIprom from Polyplus Transfection), diluted in media. The transfection mix (10% v/v of working volume) was incubated for 15 minutes before being added to the culture vessels.
Viral Vector Quantification: Viral vectors were harvested from cells 48–72 hours following transfection, depending on the product. To determine the infectious titer, we separated viral vectors present in supernatant from the cells using centrifugation, followed by quantification with flow cytometry.
Analysis: We adopted a DoE approach for efficient screening of parameters that could improve our production process. We modified up to nine parameters simultaneously in just two consecutive DoE runs using the high-throughput capabilities of the Ambr 15 microbioreactor platform and seamless integration of MODDE software. We studied the following parameters: media composition, supplements, additives, cell density, stirring speed, aeration rate/set-point, pH, and transfection-specific factors (DNA quantity, transfection reagent, and timing).
Fractional factorial screening and optimization designs were created with the MODDE software, and model coefficients and contour plot analyses were obtained to evaluate the results (data not shown).
Results
Significant Process Improvements Generated Using DoE Analysis: The data from our initial optimizations facilitated a tenfold increase in infectious titer (2). Next, we built upon our improvements through additional tests to inform a more structured approach. Experiments revealed cell density as a factor requiring further optimization (Figure 1).
We also assessed the influence of a number of media reagents and supplements, leading to additional process improvements. We combined the modifications to form the basis of a new protocol used in a second round of optimization experiments.
Our preliminary studies demonstrate that DoE models reveal critical interactions among key experimental factors and enable efficient identification of optimal process parameters. Those parameters can be tested efficiently in parallel cultivations in the Ambr 15 microbioreactor, which enables cost-effective and rapid early process optimization.
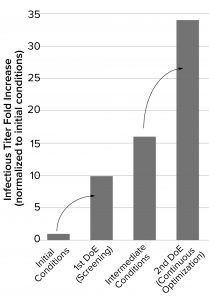
Figure 2: Summary of the infectious titers following each optimization round; the initial process was optimized through a design of experiments (DoE) screening approach (first DoE campaign) (2). Those data informed additional improvements to cell density and media components made in independent experiments (intermediate conditions). The findings were incorporated into a second round of optimization (second DoE campaign), which allowed us to fine-tune the optimal parameters.
Fine-Tuning Process Parameters Through Iterative Modeling: After identifying the initial parameters, we performed more focused validation and robustness studies. Doing so allowed us to combine and validate previous developments and refine more advanced parameters, including stirring speed, temperature, pH profile, and further media development, including addition of a transfection enhancer.
Optimizing the process parameters during this second round of screening delivered infectious titers up to 34-fold greater than the initial conditions, representing a significant improvement to the efficiency of the production process (Figure 2).
Discussion
A scalable and cost-effective production process is crucial to making gene therapies more widely accessible. This is particularly true for viral-based systems, which would benefit from the availability of stable packaging and producer cell lines for both preclinical and clinical applications. Using the high-throughput capabilities of the Ambr 15 microbioreactor and the fast-track DoE methodology offered by the MODDE software, we have screened and optimized multiple parameters rapidly to control viral vector production in the OXGENE XLenti prepackaging cell line. This approach enabled quick development of a process that increased the infectious titer by 34-fold, significantly improving our production procedures. The innovations explored here have been incorporated into our other processes, becoming a new standard for the packaging cell-line platform at OXGENE.
A key feature of the Ambr 15 microbioreactor is its ability to accommodate DoE parameters generated by the software. The advanced options are tailored to improve the DoE model iteratively as new data become available. Moreover, parameters outside the scope of the bioreactor and software (for example, our evaluation of a transfection enhancer) can be improved in tandem and integrated easily into subsequent runs while maintaining the previously identified optimal parameters. Such advances rapidly enhanced the efficiency of LV production in OXGENE’s XLenti packaging and prepackaging cell lines.
Rapid Process Optimization
Our data support use of iterative analysis and high-throughput cell culture platforms to execute rapid process optimization. We used DoE analysis to improve the LV production process in our OXGENE XLenti prepackaging cell line. We demonstrated how such an approach could accelerate therapeutic development by enabling a quick transition from preliminary research to full-scale manufacturing. Our studies explored multiple innovative ideas in a single platform, significantly reducing the time required to enhance and validate a viral vector platform by up to 50%. The insights gained from these studies have broad implications for the establishment of streamlined manufacturing of cell and gene therapies.
Acknowledgments
The authors thank Sophie Lutter (scientific marketing and communications manager, OXGENE); Katy McLaughlin (scientific content writer, Sartorius), and Amélie Boulais (head of market entry strategy for virus-based therapeutics, Sartorius) for their helpful review of this manuscript.
References
1 Sung Y, Kim, S. Recent Advances in the Development of Gene Delivery Systems. Biomaterials Research 23(1) 2019; https://doi.org/10.1186/s40824-019-0156-z.
2 Krakowiak J, Liu Q, Payne T. Rapid Packaging and Producer Cell Lines Characterization and Process Development for Viral Vector Production in Serum-Free Suspension Culture. Poster presented at the BIA conference, Edinburgh, UK, 2018).
Jakub Krakowiak is a group leader and Qian Liu is a senior group leader at OXGENE, UK. Corresponding author Lara Nascimento-Brooks is a marketing entry strategy manager at Sartorius, UK; lara.nascimento@Sartorius.com; BPSTradeMedia@Sartorius.com.
Oxford Genetics Ltd. (traded as OXGENE) is a biotechnology company specializing in design and optimization of scalable gene therapy systems. It provides technology platforms and expert solutions to accelerate the discovery, development, and biomanufacture of cell and gene therapies.
