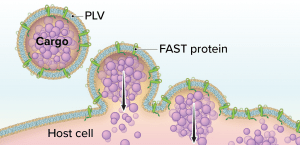
Figure 1: The Fusogenix proteolipid vehicle (PLV) platform; fusion-associated small transmembrane (FAST) proteins catalyze rapid lipid mixing between a PLV and a target cell’s plasma membrane. PLV cargo is deposited directly into the cytoplasm, bypassing the endocytic pathway.
Although vaccine platforms based on messenger RNA (mRNA) are enjoying the limelight in the wake of emergency authorizations of products from Pfizer–BioNTech and Moderna, DNA vaccines are poised to make their own commercial debuts soon. The World Health Organization (WHO) reports that six of the 48 candidate vaccines against SARS-CoV-2 that remain in clinical trials are DNA-based products, as are 14 others in preclinical study (1).
I spoke with Hong Jiang (cofounder and chief operating officer of Aegis Life, Inc.) in November 2020 to learn more about plasmid-DNA vaccines. Aegis Life spun out of Canadian biopharmaceutical company Entos Pharmaceuticals early in 2020 to pursue US-based research and development (R&D) of a pancoronavirus DNA vaccine. Jiang explains how DNA-based platforms might facilitate vaccine development and manufacture and how drug companies are addressing problems with payload delivery that beset early attempts to commercialize nucleic-acid vaccines. Drawing from 10 years in biotechnology as well as experiences in founding three companies outside the life sciences, Jiang also describes how the COVID-19 pandemic simultaneously has galvanized and impeded growth among emerging biotechnology companies.
The SARS-CoV-2 Vaccine Landscape
How would you characterize the SARS-CoV-2 vaccine landscape? With more than 200 candidate vaccines in development, clinical evaluation, and beyond (1), the landscape seems to be crowded. But we at Aegis Life are in a novel position because of the types of vaccines that we develop. Five main approaches have emerged in the first flush of COVID-19 vaccine development: viral vectors, protein subunits, inactivated viruses, attenuated viruses, and nucleic acids. Our technologies belong in the DNA vaccine segment of the nucleic-acid category. The frontrunner among DNA-based SARS-CoV-2 vaccines is Inovio’s INO-4800 candidate. What Inovio has done with that product is prove that DNA- based approaches are safe and efficacious. That will be helpful to companies such as Aegis Life. But delivery mechanisms for DNA vaccines have been cumbersome (2, 3). We intend to improve upon early designs leveraging our Fusogenix proteolipid vehicle (PLV) platform.
We have developed two DNA-based candidate vaccines (4), one of which is in preclinical evaluation. Our first targets the SARS-CoV-2 spike (S) protein. However, many vaccine developers are using that strategy, and targeting only the S protein is likely to limit vaccine efficacy and durability. Our second candidate also incorporates the virus’s envelope (E) and membrane (M) proteins.
The simplicity and elegance of the Fusogenix platform enable simultaneous targeting of all those proteins. Our scientists needed 15 years to make that technology functional, scalable, and manufacturable (5). Now, for Aegis Life, developing a vaccine or therapeutic is just a matter of identifying the right DNA plasmid, inserting it into the gene-delivery cassette, and using that to conduct animal studies — a process almost like programming software. That is why it has been straightforward for us to expand from our initial development efforts for the novel coronavirus S protein to other structural proteins.
Making a Case for DNA
Why has Aegis Life chosen to focus on DNA rather than other vaccine platforms? DNA vaccines hold several advantages over other modalities, including mRNA. RNA is inherently unstable. It’s naturally a transient molecule. Thus, vaccine developers need to adjust RNAs to make them last long enough beyond administration to take therapeutic effect. RNA also requires mechanisms for amplification and intervention from special enzymes (e.g., reverse transcriptase and RNA polymerase) that can be difficult and expensive to produce. DNA-based vaccines are simpler and have fewer technological requirements than those based on mRNA. DNA plasmids are inexpensive to produce, and DNA has greater stability than RNA. Thus, instead of fading away after a few days, administered DNA can remain in cells and continue to express target antigens for months at a time, resulting in durable protection.
Using DNA also enables quick and inexpensive manufacturing of hundreds of millions of doses per year. Aegis’s capacity for 2021 is 500 million doses, and we expect that number to grow to roughly 2 billion within the next couple of years. Because DNA is stable at room temperature, Aegis Life can ship and store DNA vaccines without need for elaborate cold-chain support. We even can lyophilize the drug substance for formulation into sublingual tablets or oral capsules. We believe that about five years from now, we will have a single-dose oral vaccine that is safe and efficacious, provides durable coverage, facilitates scalable and inexpensive manufacturing, and ships at room temperature to every corner of the world, including low-income regions in Africa, Latin America, and Southeast Asia. Fusogenix gene-delivery technology enables us to execute this vision.
You mentioned that DNA vaccines once suffered from inadequate delivery to cells. How are new candidates improving upon their predecessors? Traditionally, nucleic-acid drug products have been delivered using a viral vector (e.g., adenoassociated virus, AAV) or a cationic lipid nanoparticle (LNP). Both methods have benefits and limitations.
Viruses have evolved to permeate cell membranes using fusion proteins (e.g., the S proteins of SARS-CoV-2). Thus, viruses have adapted to merge seamlessly with our cells and deliver their genes. Pathogens such as adenovirus and AAV do that effectively and nontoxically, and vaccine developers seek to leverage that capability. But manufacturing viral vectors is expensive and resource intensive, and the process often yields small vector quantities. Vectors also can feature limited cloning capacity.
Lipid encapsulation can support large payloads of many types. An LNP can be designed to carry mRNA, small interfering RNA (siRNA), DNA, peptides, proteins, and even small molecules. Vaccine developers want such flexibility in a delivery platform. But payload delivery is a challenge with some LNPs, and cationic LNPs are not well tolerated.
Aegis Life’s Fusogenix PLV technology combines the advantages of viral vectors and LNPs while circumventing their limitations. The technology consists of a PLV that bears fusion-associated small transmembrane (FAST) proteins. Instead of relying on a positive surface charge to facilitate cellular uptake of a DNA plasmid, Fusogenix systems can merge with cells to deliver genetic cargo (Figure 1).
FAST proteins were discovered 20 years ago by Roy Duncan, who is a professor and the Killam Chair in virology at Dalhousie University in Halifax, Nova Scotia, as well as cofounder of Entos Pharmaceuticals and Aegis Life. Duncan had been studying avian and reptilian orthoreoviruses, and he noticed that such viruses merge profusely with mammalian cells. That happens, he determined after several years of study, because such orthoreoviruses bear fusion proteins that are two orders of magnitude smaller than those found on the surfaces of human viruses (6, 7).
Based on that research, Duncan along with John Lewis (cofounder and chief executive officer of Entos Pharmaceuticals and Aegis Life) and other researchers developed a fully synthetic orthoreoviral FAST protein that can be produced easily and inexpensively. Duncan, Lewis, and the rest of the Entos team also perfected the art of attaching FAST proteins to lipid surfaces and designed a suitable manufacturing process. Originally, that process entailed seven steps to integrate DNA plasmids, lipid particles, and fusion proteins. Today, the process uses only one step.
Streamlining the process required a lot of know-how and trial-and-error, but now the system is ready to produce hundreds of millions of doses per year.
Challenges with Manufacture and Scale-Up
What are you learning about vaccine development as Aegis Life’s candidates advance toward clinical trials? We are finding that neutralizing antibodies might be short lived and that much still needs to be researched about immune responses to SARS-CoV-2. Based on existing vaccines and our understanding of immunity, we know that T-cell–based responses are critical to long-term immunity. So we are designing vaccines to elicit not only strong neutralizing antibodies, but also strong T-cell–based immunity that is balanced between T helper types 1 and 2. We believe that our candidates meet such design requirements. Because our first delivers the novel coronavirus S protein intracellularly, we can stimulate a good T-cell response to it. However, because viral proteins mutate, designing a vaccine against more than one structural protein can mitigate viral resistance. That is why we have added other targets — to minimize the effects of future mutations and confer long-lasting immunity.
In terms of manufacturing and deployment, we have learned much about the global supply chain for essential materials. The pandemic has reached such a large scale that vendors of vaccine supplies — from needles to syringes, to glass vials, to just about everything else in the supply chain — could be running out of stock. That has been difficult for us. As a startup, we haven’t received hundreds of millions of dollars from the government to stockpile supplies.
Startups also face challenges in trying to find laboratories that are not backlogged to run toxicology and animal-challenge studies. Startups with limited resources get pushed down on the queue. Even though we believe that we have a superior delivery technology and a safe and highly effective vaccine, we’re facing long delays because of high demands on limited resources from larger companies. We hope that governments will make some resources available for smaller companies with innovative solutions.
Setting up fill–finish activities for clinical trials is a big challenge. Prices have risen considerably, and slots are not as available as they were before the pandemic. Such problems are crushing for startups. We wish that government agencies could make this part of the process easier for startups so that we can focus more on the science and less on the logistics of fill–finish.
Thinking Beyond the Pandemic
How might COVID-19 change vaccine development and manufacture? The push to invest in COVID-19 research has accelerated development of nucleic-acid–based medicine. The advantages of such approaches also have come to light rapidly. The obvious need is to get COVID vaccines and therapeutics up and running, but once we set up infrastructure for DNA-based drug manufacturing, we will be able to make hundreds of millions of doses of vaccines and therapeutics, and we will experience the dawn of a new generation of medicine.
The pandemic has shown that vaccine manufacturing needs a platform approach. With nucleic-acid approaches, we would not need to “reinvent the wheel” when the next pandemic arrives or when SARS-CoV-2 mutates. The wheels would be there already; we just would finetune the machine. With DNA vaccines, only a few months are needed to design payloads and test them for efficacy. It’s almost like spending a few months writing software and then testing and improving it rather than spending years searching for small molecules that provide protection. On the manufacturing side, DNA-based approaches will enable the same facilities and equipment that are used for COVID-19 vaccines to be used for DNA-based vaccines against other known and emergent pathogens. Such advances are paradigm shifts.
What else should the biopharmaceutical industry know about emerging vaccine technologies? Aegis Life has been asked over and over, “Isn’t the race for a COVID vaccine over? Aren’t you too late?” Getting a vaccine out in one year has been miraculous, but speed comes with compromises. One year of work will not yield a vaccine that can be deployed globally. The frontrunner vaccines have bought us time, but they are interim solutions with limited applications. For instance, vaccines requiring cold-chain transport and storage are impractical in many parts of the world. To be successful, those early vaccines also require enough syringes and glass vials to vaccinate 8 billion people, and such materials are in short supply already.
Eventually, we need to formulate vaccines as single-dose oral pills — products that are inexpensive and that can be shipped easily, without a cold chain, to anywhere in the world. Vaccines also should be designed to provide long-lasting immunity — minimally six to 12 months — and remain resistant to pathogen mutations. You don’t want to have to revaccinate 8 billion people a year from now because the vaccine lost its efficacy against SARS-CoV-2 because of viral mutation. That would be a huge task.
We don’t believe that the current COVID-19 vaccine frontrunners have all the answers. There still is room for innovation and improvement, and those assets can come from new generations of vaccine developers. We also hope that governments will not neglect new vaccine developers and that they instead will provide resources that facilitate time- and resource-consuming activities such as fill and finish. Something must happen to ease the logistics for companies such as ours to move into clinical trials and commercial production.
References
1 Draft Landscape of COVID-19 Candidate Vaccines. World Health Organization, 2021; https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
2 Ferraro B, et al. Clinical Applications of DNA Vaccines: Current Progress. Clin. Infect. Dis. 53(3) 2011: 296–302; https://dx.doi.org/10.1093/cid/cir334.
3 Hobernik D, Bros M. DNA Vaccines: How Far From Clinical Use? Int. J. Mol. Sci. 19(11) 2018: 3605; https://dx.doi.org/10.3390/ijms19113605.
4 Raturi A, et al. Rapid Prototyping and Immunogenicity of SARS-CoV-2 DNA Vaccine Candidates Formulated with the Fusion-Associated Small Transmembrane Protein Proteolipid Vehicle Delivery System. Under review.
5 Brown D, et al. Safe and Effective Delivery of Nucleic Acids Using Proteolipid Vehicles Formulated with Fusion-Associated Small Transmembrane Proteins. Under review.
6 Yeung MC, et al. The Cell Attachment Proteins of Type 1 and Type 3 Reovirus Are Differentially Susceptible to Trypsin and Chymotrypsin. Virology 170(1) 1989: 62–70; https://doi.org/10.1016/0042-6822(89)90352-8.
7 Duncan R, et al. Conformational and Functional Analysis of the C-Terminal Globular Head of the Reovirus Cell Attachment Protein. Virology 182(2) 1991: 810–819; https://doi.org/10.1016/0042-6822(91)90622-i.
Brian Gazaille is associate editor at BioProcess International; brian.gazaille@informa.com. Hong Jiang is cofounder and chief operating officer at Aegis Life, Inc.; hong.jiang@aegis.life.
