Andrew Lidums (NA business development manager, Parker domnick hunter) 2:30–2:55 pm
Implementing a Risk-Management–Based Approach to the Prevention of Mycoplasma Contaminations
Lidums began with a recent report from Genentech, where a 150-µm long Leptospira organism from a drainage ditch outside the facility managed to penetrate 0.1-µm filters. The company solved this problem by lowering the temperature of the filtration operation, which made the bacteria more rigid and kept them from getting through. Clearly, however, contaminations happen even at large facilities.
Mycoplasma are the smallest class of bacteria, varying in size and shape from 0.2 µm and larger. Lacking a cell wall, they can deform and squeeze through pores, making them difficult to remove with typical 0.2-µm sterilizing-grade filters. Slow-growing and difficult to detect, mycoplasma often come from agriculturally derived components in cell culture media, and some species are associated with humans, so operators can bring them into sterile suites). Low-level contamination of raw materials may not be detected but can grow in storage (e.g., Acholeplasma laidlawii can grow at refrigerated temperatures). Contamination can be costly — in corrective and preventive actions as well as product loss. Patient safety, a company’s ability to get drugs to market, and (in severe cases) the future of a manufacturing facility itself all can be at risk.
In 2009, Genzyme detected a porcine virus contamination in its Massachusetts facility. Production titers had declined over a year, but the root cause had been unknown. This contamination brought a court-appointed independent consultant acting on behalf of the US Food and Drug Administration (FDA), with significant oversight in the facility, and a fine of US$175 million. The company lost over a billion dollars in sales, and some patients without treatment filed lawsuits. That weakened Genzyme’s stock price and eventually led to its acquisition by Sanofi.
The Parenteral Drug Association (PDA) has established a mycoplasma task force of users and suppliers (Parker domnick hunter is one of five filter companies involved) to produce consensus guidelines related to filtration test parameters. Lidums described a three-part approach to defend a company against mycoplasma contamination: detection (to identify and quantify potential contamination sources), prevention (to prevent mycoplasma from entering a facility), and control (removal of suspected contaminations).
Detection methods include nucleic-acid techniques (e.g., polymerase chain reaction, PCR) and traditional microbiological assays. Lidums said that the PCR methodology is fast and detects mycoplasma genetic material but does not distinguish between such material and viable organisms. He said a major difficulty in detection comes from the fact that sample volumes are small but come from potentially large batches.
Sterile filtration for bioprocessing typically involves 0.2- to 0.22-µm filters, whereas mycoplasma require a 0.1-µm filter. PDA has already published a guideline on the former and is working on one for the latter. A subcommittee has chosen A. laidlawii grown to a small size as a model to demonstrate the greatest filter challenge. Lidums said his company offers a product (Propor MR) for mycoplasma reduction. It is a two-layer, highly asymmetric, 0.6-µm filter validated for 1010 A. laidlawii reduction or better. The company is working with customers in further retention studies using a number of organisms such as Mycoplasma faucium.
One customer found that adventitious agent in its processes and performed a challenge study on 47-mm disc filters under a range of differential pressures using a Scilog FilterTec smart pump. Filtrate was collected, and viable organisms were enumerated on dilution plates. Results showed a large drop-off in retention from 107 at about half of the initial pressure. Lidums said that illustrates a clear relationship between filtration pressure and retention. Under certain process conditions, even a 0.2-µm filter can retain mycoplasma, but not as well as a 0.1-µm filter. At a threshold pressure, the mechanical strength of the cell is overcome, so mycoplasma are deformed and pushed through. Retentivity varies by organism and process conditions. For example, some species are sterol dependent, so the presence of cholesterol, fatty acids, and so on in a process can influence that deformity and thus their ability to penetrate membranes.
Through acquisition of SciLog Parker domnick hunter brought together filtration, automation, and single-use sensor technologies to create a normal-flow filtration platform (SciFlex NFF) that allows for pressure peak maximization. The goal is to prevent pressure spikes and control design space.
One audience member asked whether mycoplasma can grow in bioreactors and potentially damage their products. Lidums said that it depends on the species. Some contaminations simply use up nutrients that should go to mammalian cells. That’s how expression titers can drop off. Sometimes mycoplasma can infect and even kill mammalian cells.
Another audience member asked whether using 0.22-µm filters for mycoplasma retention is too risky. Lidums said his company found low retention levels only with 0.2-µm filters.
View the full presentation video
René Gantier (R&D director of biopharm applications at Pall Life Sciences) 1:00–1:25 pm
Manufacturing Flexibility: At the Heart of Continuous Processing
Continuous processing could help companies move from batch manufacturing to a more flexible, disposable, and continuous production mode. Gantier discussed some technologies his company offers to enable the transition from batch to continuous processing (Cadence single-pass tangential-flow filtration (SPTFF) with in-line concentration). Also, this year Pall has acquired the BioSMB continuous chromatography technology from Tarpon Biosystems.
Continuous processing saves money on equipment, facilities, and operations. It also has the potential to increase process control and safety. Having fewer operators reduces the risk of problems and provides better control of product quality. Batch processes include many iterative steps that require extensive equipment and time at manufacturing scale. A fully continuous process would reduce the number of steps as well as the size of the equipment through intensified processing.
Pall’s philosophy is that single-use facilities are the first step toward continuous processing. Full automation then makes it possible to implement some continuous steps into hybrid batch–continuous processing (e.g., with continuous chromatography) to be able to go to the final step, which is a fully continuous process.
The company already enables fully automated cell culture through single-use technologies. And it is proposing similar solutions for downstream processing (depth filtration, virus filtration, virus inactivation, chromatography media and buffer preparation, mixing, and pH adjustments). Membrane chromatography is both disposable and automated.
For hybrid batch–continuous or even fully continuous processes, continuous chromatography is key. That’s where BioSMB technology comes in (1). Flexible in mode (e.g., flow-through or bind–elute) and chromatographic chemistry (e.g., affinity, ion exchange, or mixed mode), this technology can accommodate either resins and columns or membrane absorbers. An integrated valve cassette creates a fully disposable flow path through the many valve events needed to make a process continuous for chromatography. Prepacked columns (or membrane cassettes) and precalibrated disposable sensors enable automated, single-use processing. BioSMB technology scales up to handle the output of bioreactors with working volumes in several thousands of liters and prepacked columns up to 45 cm in diameter. For a clinical campaign based on five 2,000-L bioreactor batches with 5 g/L expression titers, a company could cut costs by half by implementing continuous chromatography.
In-line TFF concentration would be an enabling technology for continuous filtration. The Cadence system uses single-pass TFF technology in a plug-and-play design with preassembled, preflushed modules and no cassette holders. It can work in volume-reduction applications, (e.g., immediately following cell culture harvest) and final concentration steps as well as in line with chromatography columns to reduce fluid volumes and save loading time.
Gantier then proposed a continuous process using these products, from clarified cell culture through to purified product after chromatography. He presented data from an in-house experiment. Results showed a dramatic increase in specific productivity: multiplying the amount of product that is processed in a given time by 10. The experimental process ran for over 20 hours, producing results of stable quality. Such downstream productivities could make it less important to increase cell-culture performance, ultimately reducing time to market.
For bioreactors and the depth filtration, Gantier explained, Pall offers no continuous process for now, but it does provide automated single-use technology. “So this is what we propose for now: a fully single-use, automated, hybrid batch–continuous process. We can deliver this right now, and of course in R&D we are working toward further solutions for a fully continuous process.”
When asked about bioreactor bioburden controls, Gantier admitted that it would be a challenge. “You can implement some bioburden control filters in a process before and after the chromatography.” But a fully continuous process would not include additions or removals of materials during processing, potentially facilitating bioburden control. For hybrid solutions, bioburden control would require additional filters.
Reference
1 Bisschops M, et al. Single-Use, Continuous-Countercurrent, Multicolumn Chromatography. BioProcess Int. 7(6) 2009: 18–23.
View the full presentation video
Rudolf Pavlik (director of product development for process equipment at Thermo Fisher Scientific ASI) 1:30–1:55 pm
An Application Presentation for the imPULSE MDS
With the expanding global footprint of biomanufacturing — and specifically of single-use products used in such processes — technologies that enable safe and efficient processes are increasing in importance. Pavlik presented a few real-world scenarios that used his company’s imPULSE mixing, docking, and shipping (MDS) system in biopharmaceutical manufacturing. The system combines a motor-mounted docking station with a vessel designed for both mixing and shipping. Valuable contents can be mixed directly in their shipping containers, which can then be shipped elsewhere and later remixed/reconstituted.
Pavlik discussed imPULSE mixers, which scale from 30 L to 5,000 L using a unique and customizable technology. “We can customize the systems from a hardware perspective as well as the operating system perspective,” he said. These mixers can keep mixing contents until they are empty, improving the efficiency of evacuation processes. The mixing drive is located on the bottom of each unit, making them fit well into tight spaces, especially at larger scales (3,000–5,000 L).
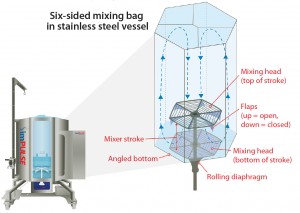
A mixing head with silicon flaps is part of the single- use bioprocess container and attached to the drive, which provides a vertical (up and down) motion. The speed and stroke of such motion are fully variable. The silicon flaps on the mixing head open and close. On the upstroke, they open to allow the head to pass freely through the tank. On the downstroke, they close to create a solid surface, which then displaces the column of liquid between the vessel’s inside shell and the outside diameter of the mixing head itself.
Robust ≤2-Hz mixing (2 cycles/second) handles powders and liquids of different viscosities. A standard design docks a single-use bag in the mixing vessel with a couple of outlets on the bottom (one typically for system evacuation, the other for recirculation of media). Two upper inlets are typically used for recirculation and addition of materials. Some customers have ports added on the side for conventional or disposable probes. Scalability comes from the volume of displacement under the mixing head and the overall volume of the tank: The ratio is always the same, for 30 L up to 5,000 L.
Pavlik went on to describe how some imPULSE mixers have been customized for specific applications. Clients wanted to reduce the number of fluid transfers and improve process flows. First, a client made a master batch (2,000–3,000 L) and transferred it into stainless steel tanks (30–50 L each), which were then transported to different locations around the world. Receivers drained the tanks and put their contents into mixing systems for reconstitution and final processing.
Pavlik’s company suggested an imPULSE mixing– docking–shipping station on both the shipping and receiving ends so that tanks could be decoupled and transported:
- A master batch is transferred into single-use bioprocessing containers inside disposable 50-L drums.
- Sampling-ports allow users to pull samples for testing.
- Receiving clerks place shipped drums into their own docking station, a stainless steel system that reconstitutes fluids for final processing.
That solution eliminated transfer into and out of shipping containers, which is a source of contamination risk. Costs were reduced, and overall timeframes were shortened. Pavlik highlighted the lack of a secondary filtration process after reconstitution. The supplier has also tested imPULSE systems for particulates (USP chapter <788>) as well as drop and transportation integrity.
Pavlik concluded with a physical demonstration of an actual imPULSE system.
View the full presentation video
Michelet Dorceus (bioprocess application scientist, Eppendorf North America) 2:00–2:25 pm
Large-Scale Production of Human Mesenchymal Stem Cells in BioBLU 5c Single-Use Vessels
Over the past few years, stem-cell–based regenerative medicine has been a challenge in the scientific community. Such therapies have the potential to revolutionize human disease treatment. Although successful expansion of mesenchymal stem cells (MSCs) is well established in vitro, large-scale production remains a bottleneck. Dorceus shared a related application generated at his company.
MSCs are adult stem cells of stromal origin. They provide connective tissue to many parts of a human body, such as prostate, bone marrow, adipose tissue, and ovaries. These stem cells are involved in more than 400 clinical trials (www.clinicaltrials.gov). Among adult stem cells, MSCs are the most sturdy and show the most potential in clinical testing. They have potential application in treatment of baldness, blindness, diabetes, Parkinson’s disease, Alzheimer’s, and stroke, just to name a few.
Materials and Methods: Eppendorf used adipose-derived MSCs from ATCC and a basal medium supplemented with MSC growth components. For the first experiment, the team used SoloHill polystyrene microcarriers from Pall, and for the second experiment they used collagen-coated microcarriers. For analysis they used a NucleoCounter NC-100 cell counter from Chemometic and a Vi-Cell XR viability analyzer from Beckman Coulter. They chose a bench-top CelliGen BLU stirred-tank bioreactor and controller from New Brunswick Scientific (now part of Eppendorf) for its flexibility and range of options available (e.g., low- and high-flow gas delivery; optical and standard pH control; batch, fed-batch, and perfusion).
To produce one therapeutic dose of stem cells, the best vessel was a 5-L BioBLU 5c. MSCs are very sensitive to shearing, so gentle agitation (at lower rpm) is preferred whenever possible. So the team found the slowest possible agitation rate to use without compromising mixing efficiency: 25–35 rpm. They prepared an inoculum in a New Brunswick S41i CO2 incubator–shaker, using microcarriers to do so for the first time in the industry with a shake flask inside an incubator.
Results: The polystyrene microcarrier culture reached a cell-density peak of 400,000 and ran out of glucose while producing lactate, as expected. The culture was fed through a 25% media exchange on days five and 15. Although cell density had increased almost sevenfold, they were not satisfied with those first results because they wanted to obtain 1.6 billion cells. So they changed a couple of parameters and tried again using collagen- coated microcarriers, which some literature suggests better supports MSCs. Then, cell density peaked at 2.5 × 105 with a 50% media exchange every four days. Culture was terminated on day 18 (with 98% cell viability), at which point the team believed that continued media exchange could extend the cell density even further.
Next they tested the MSC cell properties through immunoassay analysis. The cells expressed CD 44 and CD 90 markers. PCR results were positive for all stem cell markers: Oct 3/4, Sox 2, CD 105, CD 90, and CD 44. Results of two differentiation assays (adipogenic and osteogenic) were positive.
The team also monitored cell counts and viabilities. Early readings correlated from both instruments. But the cell counter’s capabilities were limited at higher cell densities. So the team used the viability analyzer to correct their growth profile results, which gave a total of about 1.6 billion cells overall.
Discussion: Dorceus concluded that his team had produced more than the goal of a 1 billion MSC therapeutic dose in a BioBLU 5c single-use vessel, retaining the necessary differentiation and multipotency properties of those cells in a current good manufacturing practice (CGMP) compliant environment. This shows general applicability of the CelliGen BLU system and the BioBLU vessel for large-scale adult stem cells. He said the company has more data supporting scale-up to a 50-L single-use vessel and larger. He described Eppendorf’s offerings for cell therapy and other biologics R&D, including the DASGIP system; process development; and large-scale GMP production.
View the full presentation video
Jim Furey (general manager, PendoTech) 3:00–3:25 pm
Qualification and Control System Integration of Single Use In-line Sensors
Furey overviewed his company and the main sensors used for in-line monitoring of bioprocesses. He described user requirements, monitor tesing, and control system integration. The primary in-line measurements for bioprocess monitoring control are pressure, temperature, conductivity, absorbance (e.g., UV), turbidity, pH, and flow. Sensor design requirements relate to cost and type of use. One way to lower cost is to minimize product-contact components and reuse electronics.
In choosing among products on the market, users have their own requirements related to product-contact materials, performance, sensor manufacturing, control- system integration, regulations, and compatibility. Requirements for sensory accuracy, range, and other parameters are different for each process. Some companies integrate sensors into high-level control systems. Regulations help users determine what they need.
Sensor materials must be safe for bioprocessing. For biocompatibility and toxicology, most users refer to USP class 6 for medical devices, which has more stringent requirements than ISO 109A3-1. Companies want to prevent transmissible spongiform encephalopathy (TSE) exposure per EMA 410 guidelines. In some cases, compatibility with sterilizing gamma radiation is important. Disposable sensors must be assembled in a clean manufacturing environment and shouldn’t require calibration on the users’ part. Suppliers need to have good quality systems in place with traceability and standard operating procedures (SOPs).
Components must be compatible with conditions and materials in a given process. Chemical compatibility, leachables and extractables, and physical compatibility with process parameters are all important. Even if a sensor doesn’t need to operate well at certain extremes, it cannot fail and cause accidental product exposure. Compatibility with sterilization and bioburden-reduction techniques (e.g., autoclaving and radiation) also must be established.
No single product will meet every user’s process requirements. Suppliers target a range of performance parameters to meet the requirements of many applications. For example, PendoTech uses USP class 6 materials that meet EMA 410 guidelines. Each sensor is tested for accuracy and electrical continuity, with no calibration required, and all units are cleanroom manufactured in an FDA-registered, ISO 13405 facility. PendoTech pressure sensor chips are microfabricated to tight tolerances and tested to perform within those set tolerances, making calibration unnecessary. For some applications (e.g., viral clearance), regulators may want to see calibration data, so the company offers NIST-traceable documentation for individual sensors if needed.
Single-use sensors make indirect measurements: They measure parameters that correlate to the measurement of interest. For example, pressure sensors measure an excitation voltage and require electronics to read and transmit their results. Furey mentioned his company’s Pressuremat monitor, which has inputs for sensors and outputs to interface with higher-level control systems or personal computers. With different electronics, different sensors can perform differently, and the electronics themselves can present limitations.
Furey presented a user-qualification case study of an absorbance monitor, a type that does not contact process fluid but rather shines a light through it. He explained the importance of that light’s path length (e.g., 1 cm) and that it must be precise. These instruments are often used in filtration and chromatography unit applications, where the sensor provides a window, and the monitor is key to its performance. A simple photometer is qualified separately through a comparative performance evaluation. One customer compared a single-use UV sensor from PendoTech with a traditional instrument in several runs, with good results. Other qualification methods for monitors (separate from their sensors) use millivolts for pressure monitors, resistance simulation for temperature and conductivity monitors, and analytical standards for absorbance monitors.
Furey said that very few standard communication options are available for integrating these sensor–monitor systems with programmable logic controllers (PLCs), Delta V (Emerson Process Management), and other control systems. “Surprisingly,” he said, “analog signals are still very common because they’re well defined, easy to use, and low cost.” But digital technology is gaining prominence despite its lack of standards.
Single-use sensors are becoming a robust technology for GMP processes. Suppliers can provide qualification data and validation guides. In some applications, performance could even be improved by switching from traditional instrumentation. Disposable sensors allow for real-time monitoring, control, and trending of process performance.
An audience member asked why the industry has been slow in adopting wireless communications. Furey said it has been slow even to adopt single-use sensing technology at all, but that wireless should be the next evolution. He cautioned that power issues can arise, even with battery back-ups, as well as potential crosstalk.
View the full presentation video
Paul M. Priebe (director of fluid management technologies, Sartorius Stedim North America) 3:30–3:55 pm
Innovative Raw Material Management and Product Design Strategy for Enhanced Quality, Assurance of Supply, Validation, and Change Control of Single-Use Systems
Priebe spoke of market dynamics and challenges, then described a plastic film development strategy that addressed them. He said that implementation of single-use technology is growing at a greater pace than the overall biotechnology market. As biologics sponsor companies scale their technologies up to commercial production, their projects drive that market growth.
Priebe also described an industry in transition. A decade or two ago, disposables were used primarily in media and buffer preparation. But now they have penetrated whole bioprocesses. The resulting supply chain, he pointed out, has become very complex. The number of suppliers a single company can depend on is proliferating. Pointing to a January 2003 explosion at a West Pharmaceuticals facility, Priebe said that such incidents can present a business continuity risk — in that case, related to elastomeric stoppers for container–closures. Another learning experience for the industry came in October 2010, when a supplier of polypropylene (used across industry for component molding) announced a formulation change. That brought to light the importance of change control and notification.
Finally, Priebe pointed to a March 2013 paper published by Amgen, which had found that a secondary degradant of one antioxidant used in polyethylene film had leached into cell culture media and ultimately killed cells in the seed train. Because of the very low trace levels of extractables/leachables in the process, that complex investigation took several years to complete. “There are still unknown unknowns,” he said, “about the interaction between bioprocesses and polymers.” The secondary degradation product Amgen identified came about partly due to bag handling. Recalling the old bioprocess adage that “the process is the product,” Priebe said that it holds true for plastics as well.
Few suppliers are committed to the needs of the bioprocessing market — even as users and vendors are working together toward developing standards for single-use systems. Implementation of single-use systems clearly remains a challenge for biomanufacturers, but many organizations are putting energy into solving some of these problems. This creates a requirement for product improvement to feed the growth rate of the industry.
Priebe went on to describe his company’s development of a new polymer film (Flexsafe) as a means of addressing some of those challenges. Product development, he said, comes down to translating user requirements into technical specifications. His company collaborated with two others: a film–extrusion partner and a resin manufacturer. That allowed control of bag properties such as flexibility and strength through controlling properties of the film the bags are made of, with all parties involved in discussions over leachables and extractables, and so on.
Sartorius Stedim Biotech took a quality by design (QbD) approach to developing its new family of single-use products. That’s about understanding the manufacturing process, said Priebe, and how variations in that process can affect the characteristics of its end product. This allows for continuous improvement. For example, referring back to the Amgen incident, Priebe said that SSB wanted to control and maintain all critical parameters of its plastic film. That involved studying different parameters of the extrusion process to develop a design space within which the results would be robust and reproducible. Then SSB evaluated the critical process parameters (CPPs) of this film in relation to cell growth.
Assurance of supply, said Priebe, comes from a combination of business continuity, consistency of quality, and change control. Each step of single-use system manufacturing needs to be controlled: from plastic resins to films to bags and components, assembly, and sterilization. SSB vertically integrated partners, suppliers, and contract manufacturers into that process. Each step is subject to quality assurance, assurance of supply (business continuity), and change control.
Instead of buying stock resins, Priebe’s company has them made to its own specifications and recipe. “We’re only using mainstream polymers and additives,” he said, “that are widely available and will be around for the foreseeable future.” Those specifications and recipe are controlled from batch to batch through a quality agreement with the contract manufacturing organization (CMO). And the same elements come into play for change control. For business continuity, if the current CMO decides to terminate its contract, a “last-time buy” option allows SSB a period of multiple years to bring in another. Meanwhile, it is qualifying a second extruder and maintaining a rolling inventory. Final assembly is its own operation, with secondary manufacturing sites and a third site currently in construction — all with redundant capabilities and duplicate quality systems and equipment. Finally, contract service providers gamma irradiate components for sterilization, with a minimum of two qualified suppliers for each SSB manufacturing site. The company takes a six- sigma approach to sterility assurance.
View the full presentation video