Combined advances in molecular biology, cell biology, and genomics have led to a wealth of new information about cellular processes. A growing understanding of the fundamentals of cell biology is now being translated into products that use an approach to exert a biological effect that is different from that of most biologicals currently on the market.
To date, most biological products consist of highly purified proteins with a specific activity that alleviates or stops the symptoms of a certain condition. Increasingly, however, the “next generation” biologics exploit knowledge gained in cell biology to directly interact with specific cell types or receptors. Such highly specific interaction can direct them to activate or inhibit the signaling cascade of a specific pathway, generating a specific local or systemic biological effect. Another application would be in exploiting metabolic capabilities of an organism or occupying a niche that would otherwise be occupied by a pathogen.
What most of these second-generation products have in common is that the dose needed to produce a clinical effect is generally an order of magnitude lower than for current highly purified biologicals.
Therefore, next-generation biologicals will not necessarily require the use of large steel tanks in production, but rather fairly small, single-use production vessels of perhaps a couple hundred to 1,000 L working volume. This not only reduces the cost of goods for such products, but it also enables a high degree of production flexibility.
Although the “purification” of live organisms is generally straightforward, the fact that such organisms typically must be lyophilized to retain their viability does add to the complexity of their manufacture. For example, because the total processing time of a batch affects the viability of the cells, it is preferable to have both production and lyophilization happen at one site.
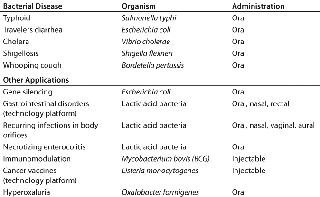
The use of live biologicals is not new. Table 1 lists a number of classical products. The first was the cowpox virus, after which many followed. The fight against polio would not have been successful without the live, oral vaccine. Typically, the oral vaccine contains 100-fold fewer virus particles than the inactivated vaccine contains. The difference in cell content of an inactivated typhoid and an oral attenuated typhoid vaccine is on the same order of magnitude.
Table 1: Overview of currently registered live agents for human use
To illustrate the effect such a difference in dosage has on the costs and the scale of production, an example from the vaccine field is most clear. The Netherlands Vaccine Institute (NVI) historically produced inactivated polio vaccine for the Dutch population of 16 million. As part of a joint development project with China in the 1990s, the NVI developed and transferred the process for manufacturing oral polio vaccine to China with only a twofold increase in scale. That was sufficient to supply China with vaccine for its 1.3 billion inhabitants.
Live Agents in Development for Human UseAs mentioned, live biologicals have been used in the vaccine field for many years. Logically, a number of such products under development are live vaccines that offer a clear advantage over existing products on the market (Table 2). For example, the typhoid vaccine currently being developed by Emergent Biosolutions Inc. (www.emergentbiosolutions.com) has been reported as capable of eliciting a protective immune response after one dose, whereas three to four doses of the registered live typhoid vaccine are needed to achieve the same level of protection.
Table 2: Some live agents for human use that are in development
For whooping cough, the situation is similar. Live vaccines are expected to confer protective immunity in humans after a single dose (1), although other considerations play a role as well. Most hospitalizations for whooping cough occur when infants are zero to five months old, which is when the disease can be life-threatening. The most relevant advantage of a live vaccine is that it can probably be administered to newborns (2), whereas all registered acellular injectable vaccines can be applied only after two to three months, with protection being complete after five to six months.
If the attenuation of a live pertussis vaccine is chosen well, it should not give rise to many side effects. Currently registered acellular vaccines cause side effects that increase with each dose. That makes it unlikely that such vaccines can be given to adults who have received the full series of acellular vaccine as infants. Therefore, the third advantage of a live pertussis vaccine with few side effects would be that it can be used in adults. That huge market is currently served only partially, and a live product would cost but a fraction of the price of a purified injectable vaccine.
Rather than developing a single product, such as a live vaccine, many companies have developed or are developing live microbial-based platform technologies that can be used for a range of afflictions. For example, ActogeniX (www.actogenix.com) has developed a platform technology that uses lactic acid bacteria to deliver various biologicals to the gastrointestinal tract, where the active components are produced at low levels over an extended period. In this case, there is no other treatment that quite matches the delivery and “slow release” of the active biologicals delivered by those lactic acid bacteria in the gut. Using the same biologicals in the traditional injectable form would cause severe side effects and thus would not be feasible as a treatment. The costs of growing and lyophilizing bacteria that excrete a biological are small compared with those of purifying the same biological to the point that it can be injected. Also, a repeated oral dosage form is always considered more user friendly than repeated parenteral administration.
An additional advantage of the ActogeniX technology platform is that other products can be prepared using exactly the same production process with only a few different product-specific QC assays. So the costs for process development of a later product can be greatly reduced when the cultivation, harvest procedure, and even the lyophilization buffer and lyocycle can be assumed to be identical to those of the first product.
A live biological is more difficult to produce if it has to be injected. This is the case for products developed by Advaxis (www.advaxis.com). Its technology platform uses the unique properties of Listeria and listeriolysin to direct a host’s immune response toward the destruction of cancer cells. Even though the number of Listeria cells administered to human patients would be quite small (109 to 1010 per dose), their effects are quite pronounced (3). The results look promising apart from the side effects, which are to be expected, and the fact that the first human study was not designed for efficacy. Also, an interesting observation is that the live Listeria can be administered to people who are quite sick and still elicit the appropriate immune response.
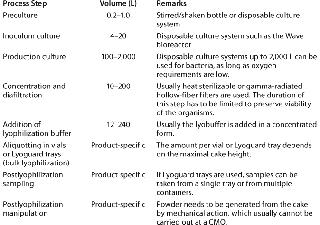
Production Processes and ProblemsThe typical production process for a live biological is fairly simple (Table 3). The organism is cultured in a steel tank or a disposable culture system. Usually the cells are concentrated using disposable microfiltration equipment, then diafiltered against a buffer or saline solution, after which a lyophilization buffer is added and the resulting formulation is freeze dried.
Table 3: Generic production process for live biologicals
The requirements for oral products allow some contaminating bacteria and yeasts to be present in an oral formulation alongside the desired live organism, whereas certain species such as salmonella or coliforms must be completely absent. However, if the formulation is to be administered parenterally, no other microbes can be present. Proving that is as difficult as proving that there is no needle in the proverbial haystack. Thus, a suitable “magnet” is required to verify the “needle’s” presence or absence.
Theoretically, it should be feasible to grow contaminating organisms on media that do not support growth of the desired organism, thus providing a means of testing for their presence. Establishing such media is time consuming, and their success is uncertain. Instead, it is wise to incorporate a metabolic marker that allows easy discrimination of a desirable organism from undesirable contaminants. Also, for biosafety reasons, it is wise to add a metabolic marker to prevent such organisms from spreading into the environment.
Aside from showing that a sample (a very small portion of the entire batch) is free from contaminating organisms, a company also must provide data showing that production of an injectable biological is operated in a fully contained system. Such data can be generated by carrying out the entire process with media that support growth of any organism. All containers, filters, and so on can then be incubated to show that no organisms are present. This approach would be far more feasible for disposable systems than for stainless steel production systems.
Before lyophilization, a live biological is aliquotted into either vials or a bulk lyophilization container, depending on the final dosage form. For the latter, Lyoguard trays present a useful addition to existing lyophilization containers. Because theGore membrane of these trays is impermeable to viruses and bacteria, the trays can be viewed as a closed system once they have been filled and closed. This is a very relevant aspect of lyophilizing large amounts of live bacteria (with containment in a class A in B environment) as well as product safety (from using a closed lyophilization system).
Most oral formulations have to be released into the intestinal tract to exert their biological effects, which means that freeze-dried cakes in trays must end up in capsules. First, the cakes are crushed and sieved into a homogeneous powder, usually with excipient(s) added. The lyophilized bacteria are encapsulated, and the capsules are coated, in turn, with a polymer that protects them during passage through the acidic stomach environment.
The encapsulation process is not trivial. Because all lyophilized formulations are hygroscopic, these powders should be handled only in low-humidity environments; otherwise, absorbed moisture from the environment will lower the glass-transition temperature and lessen the stability of the bacteria. Low humidity causes static electricity, which in turn makes a lyophilized powder difficult to handle, which poses a biocontamination risk. There is always some absorption of moisture from the environment, so any manipulations of freeze-dried powders should be brief. Lyophilization buffer and cycle development should thus produce a formulation with a high glass-transition temperature. This way, some moisture can be absorbed without endangering the stability of the live organisms. Unfortunately many formulations include sucrose, which has an inherently low glass-transition temperature.
Key Drivers for Live Agents Under DevelopmentFirst-generation biologicals focused on purification of biologically active molecules to target. In other words, a lot of the “low-hanging fruit” has already been picked. Second-generation biologicals necessarily aim at developing cures for diseases that are more difficult to treat but also are less common. The latter particularly leads to a much smaller annual number of doses needed for such biologicals than the annual number of doses required for diseases targeted by the first generation of biologicals.
From an “active ingredient” point of view, the possibilities of live organisms directly interacting with specific cells or receptors can, of course, never be achieved by a single molecule alone. Because of this specificity, the human dose for a live-organism biologic is generally quite low. For example, at 109 cells/dose, a mere 100-L culture will probably yield approximately 100,000 doses or more of the Advaxis cancer treatment, which is probably sufficient to handle all cases in a country the size of the United Kingdom.
A definite advantage of live biologicals is that many can be prepared using fairly small-scale, disposable equipment. From a production perspective, the comparatively low annual requirements of these biologicals make dedicated production facilities uneconomical. Therefore, given that these products are made in multipurpose facilities, validation efforts are simplified significantly when disposable equipment is used.
Live agents are increasingly attractive as therapeutic or prophylactic agents for a number of reasons. Although only a handful of products have moved beyond phase 3 clinical trials, it is clear that live biologicals will transform from a niche product to mainstream. Currently, not many contract manufacturing organizations (CMOs) are willing to allow live organisms in their fill–finish suites, nor are there many CMOs with fermentation and lyophilization capacity located in the same facility. However, the chances of finding a CMO with the right experience to produce a live biological are still better than finding that proverbial needle in the haystack.