Cellular therapy continues to expand and gain momentum, as evidenced by the growing number of companies and clinical trials in the field each year. Early potential therapies were developed solely by investigators without communication or input from manufacturing experts. That communication gap led to numerous setbacks as potential products were developed without roadmaps for feasible manufacturing scale-up (or scale-out). Contributions from members of the cell therapy community over the past few years have significantly improved the situation in the form of peer-reviewed articles and organized meetings (1,2,3). Now, early stage development teams and clinical investigators are much more aware of impending manufacturing scaling challenges as future products move through clinical stages. Their cross-functional collaboration will undoubtedly lead to higher chances for product success.
Cell therapy can be considered a hybrid of disciplines and processes. Although its origin can be traced to blood transfusions and bone-marrow transplantations, the field now includes multiple therapeutic platforms that include biopharmaceuticals, biologics, and medical devices. That blend of disciplines is important to diversity and growth, but it presents a number of challenges for process and product development. Product development in an academic laboratory or clinical setting is very different from that in industry or “big pharma.” Disposables, processes, supporting regulations, and so on are all vastly different in those settings. Typically, each discipline will interact at some point during cell therapy process development.
Even with growing cross-functional awareness and improvements, significant challenges remain as groups look to navigate scaling hurdles from lab bench to bedside. Simply considering the move from an open step to a closed step can be challenging (Figure 1). Single-use, ready-to-use, disposable components and systems play a key role. Because such products offer a number of benefits, they are commonly used in bioprocessing and cell therapy applications (2,3,4). Among other items, disposables provide the necessary flexibility in cost and design to support variation in development and manufacturing among cell therapy products. Single-use technology represents a bridge between small-scale development and larger-scale (either out or up) bioprocessing and manufacturing requirements.
Single-use components are commonly used in cell therapy processing, but the nature of disposables and processes used by academic culture laboratories, clinics, and industry settings are considerably different. The types of disposables used and the processing steps applied depend highly on the product’s origin (Table 1). For example, processing components and equipment used in a blood transfusion laboratory typically consist of transfer sets, collection and storage bags, syringes, Luer connections, and so on. Standard cell culture bioprocessing laboratories typically use tissue culture dishes, plates, flasks, and pipettes. As a product moves along its development scale from laboratory to investigational new drug (IND) and subsequent clinical stages, associated bioprocessing steps continue to evolve to accommodate increased regulatory scrutiny and scale. The bench-to-bedside progression, whether scaling up or out, requires a number of changes along the way. Understanding those processing steps and developing a scalable system to accommodate them is important to product success.
Table 1: Differences in cell therapy processing are influenced by the product development setting.
The benefits of implementing single-use technology into manufacturing processes have been discussed at length, especially as they relate to the biopharmaceutical industry (4,5,6). The concept of a closed-system kit for cell therapy processing can offer many of the same benefits while providing custom flexibility. Given the unique nature of each therapy in development, flexibility is a key requirement. While the biopharmaceutical industry navigates a transition to disposable technology from stainless steel, the cell therapy industry has been quite familiar and generally dependent on single-use components. As cell therapy products evolve from preclinical through clinical phases, development of scalable manufacturing processes becomes necessary. Some benefits of a custom single-use kit may include flexibility, scalability, bench to bedside application, minimized risk due to fewer manual/open steps, batch-to-batch consistency, a feasible transition to automation, and reduced production time. This provides a cost-effective solution for early stage, small-scale applications.
In early stages, cell therapy batch sizes are often small — especially for autologous products (even at commercial scales). Furthermore, the optimal product dosage has not been determined yet, nor have processing steps been optimized. Although open processing steps can be performed (typically) in a biological safety cabinet (BSC) early on, they become more challenging to accommodate as scale increases. Open steps depend on BSCs or even cleanrooms. Customized, closed, single-use kits are prepared and presterilized to reduce the number of open steps and minimize the risk of contamination.
In a typical process, media or other reagents are filtered, tubing sets are assembled and sterilized, and cells are transferred or washed — all tasks often performed by laboratory personnel. Not only does that require time, but it can also lead to significant variation and error. For subsequent clinical application, products may require a final processing step just before patient administration. A custom, preassembled, single-use kit can satisfy each requirement and ensure batch-to-batch process consistency. Single-use solutions can improve process consistency and product success when developed and implemented at the right time.
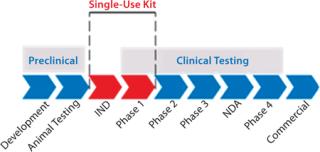
When to Close the ProcessCell therapy processing steps become inherently less flexible as a potential product moves through its clinical phases. Raw materials, disposables, and processes can be revised in early testing phases, but as a product enters phase 3 clinical trials
its processes and components must be set to ensure consistency. Implementing a single-use disposable kit is possible as a product enters Phase 3 trials, ultimately it is best to do so at an earlier stage — ideally in Phase 1 (Figure 2). Implementing before or after phase 1 testing depends on a given manufacturing process and a cell therapy’s intended final delivery.
Preclinical stages (including early product development and subsequent animal testing) are very early in a cell therapy product’s life cycle and do not require the same process scrutiny as do clinical phases. Depending on process complexity, implementing a custom single-use kit before initiating phase 1 clinical testing can work. Such kits are not necessarily meant to encompass an entire manufacturing process from start to finish, especially not in early stages. Early models may be developed to address specific parts of a process. Incorporating single-use kits at an early stage may
-
Reduce the number of manual handling steps and minimize opportunities for product contamination
-
Enable an efficient and reproducible process for transfer of product and subsequent delivery to patients during clinical testing
-
Provide a thorough understanding of process steps and potential gaps
-
Facilitate a plug-and-play process that can be more readily scaled out/up for later-stage clinical testing and commercial-scale automation.
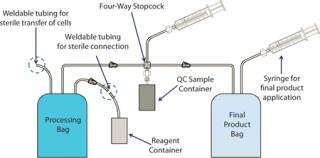
Some kits that are commonly developed and manufactured for cell therapies address final processing and delivery to patients. These custom kits regularly incorporate components to allow for transfer, storage, washing, and subsequent bedside delivery of cells all within a closed, sterile system (Figure 3). That reduces the number of manual steps and opportunities for contamination — and in turn provides a nice solution for “bedside” administration. Development of a kit also presents an opportune time for companies to think critically about their processes and how well they can adapt to larger-scale requirements.
In Figure 3’s generic example, a customized kit provides a closed system for final cell processing, with an aliquot bag for quality control, and dispensing and final delivery.Some manual steps are eliminated, and the entire process is closed. Compared with an open, manual process, it has been simplified with fewer, safer steps, and can be completed at bedside. Implementing this kit early on facilitates larger-scale development later. Additionally, the kits can be adapted to automation technology for addressing future commercial applications.
Assembling a KitClosed, single-use kits can be assembled in a number of ways, but all require a thorough understanding of process steps and critical components. Standard off-the-shelf disposable kits are available, but most are specific to certain equipment and used for stem-cell collection and processing. Because of the unique nature of each process and product, standard disposable products are often not ideal; customization is often required to meet efficiency and/or output needs.
During early clinical testing stages, companies will exploit abundantly available off-the-shelf disposable components to assemble their own custom kit(s). Technicians and laboratory personnel often take different parts and pieces and “manufacture” their own custom kit(s) internally. Cost savings is a major reason for that, but another contributing factor is a lack of commercially available products designed to support a specific cell therapy process. And in-house customization can be effective and provide a temporary solution, but it probably is not the best practice. Internal assembly of a custom kit requires time and labor. Sterilization of either an entire kit or its components also is essential. Assembly can often lack the necessary control and validation needed for current good manufacturing practice (CGMP) compliant product development. Ultimately, the kit design may not be scalable, which can translate into delaying subsequent clinical testing and future product commercialization.
Cost of development and cost of goods (CoG) are significant concerns for cell therapies, especially as early phase clinical testing begins, leading companies to rely on their internal capabilities. A negative impact on the final product can be a natural consequence. Inconsistencies in process development of smaller batch sizes for early stages will manifest when larger batches are required. Although manufacturing scale-up concerns are obvious and well documented (1,2,3), product efficacy and safety also can be compromised and lead to poor clinical results.
Alternatively, cell therapy groups can invest in the expertise of available commercial manufacturers. Single-use technology vendors are a logical choice for designing and assembling custom, closed-system solutions. These companies are experts when it comes to disposables, and some provide added benefits such as custom component and kit development, assembly validation, packaging, and sterilization. Many have product development and engineering teams that can collaboratively work with cell therapy product and process development groups to design safe, effective, and scalable solutions. Initiating a relationship early in product development facilitates later transitions and lightens scrutiny during regulatory review. In fact, a validated, closed, and presterilized process is considered by regulators and reviewers to be a beneficial feature before initiating clinical trials.
Potential up-front costs of having such a custom single-use kit developed and manufactured can be a deterrent when companies consider implementation at early stages. And additional hurdles may be encountered. Batch sizes are typically very small in phase 1 (100–200 units), and few commercial manufacturers are willing to build custom products before many thousands of units are needed. Given those perceived challenges and limitations, many cell therapy groups simply choose to rely on internal capabilities for early phase product manufacturing. But that can be detrimental to early stage success and long-term scalability alike. To address those hurdles, commercial single-use manufacturers are expanding their off-the-shelf disposable offerings for cell therapy development. And manufacturers such as Charter Medical are open to developing custom components and kits for smaller-scale applications.
Working Together for the FutureCellular therapy is an exciting field with significant promise for the future. Success will be measured not only by development of effective products and enabling components, but also collaboration of groups working closely together to build scalable solutions. The variability and complexity encountered with each cell therapy process represents long-term scalability risk. Products and processes are often developed based on their originating settings, then subsequently rely upon internal expertise for small-scale clinical testing. The results can lack necessary scalability for larger batch sizes when processing steps are not ideally suited for large-scale clinical environments. Developing and implementing closed-system single-use kits early in cell therapy development provides a safe and effective solution for long-term product success.
Author Details
Dominic Clarke, PhD, is the cellular therapy and cryogenic storage product manager at Charter Medical, 3948-A Westpoint Boulevard, Winston-Salem, NC 27103; 1-336-714-4217;