Pierre Fabre, the second largest independent pharmaceutical group in France, recently opened a new facility to expand its monoclonal antibody (MAb) production for clinical supply. The Antibody Biotechnology Unit (ABU) facility was designed to provide needed flexibility for adapting to various process and capacity changes, so it includes state-of-the-art single-use technologies. The facility was also built with sustainability in mind to minimize the company’s environmental footprint. The company integrated this plant into an existing antibody research and development (R&D) center, the Pierre Fabre Immunology Centre (CIPF), near Lyon in France.
Conceptual design of the ABU facility began in late 2008. The work phase began in March 2010, and construction was completed in only 17 months. The facility has been operating under full good manufacturing practice (GMP) status since September 2012. Here we describe the planning and design of this facility, highlighting its innovative process technologies and environmentally sustainable approach.
PRODUCT FOCUS: BIOLOGICS (ANTIBODIES)
WHO SHOULD READ: FACILITIES, OPERATIONS/MANUFACTURING, AND PRODUCT DEVELOPMENT MANAGERS
KEYWORDS: DISPOSABLES, FACILITY DESIGN AND ENGINEERING, UPSTREAM AND DOWNSTREAM PROCESSING, ENVIRONMENTAL CONTROL AND SUSTAINABILITY
LEVEL: INTERMEDIATE
With the support of Biopharm Services Ltd., the Pierre Fabre project team set a six-week timeframe to develop design layout scenarios and evaluate technology options. Key aspects we considered were as follows:
-
Basing processes on mammalian cell culture
-
Maximizing the use of disposables and skid-based processing equipment to minimize and phase-in upfront investments
-
Identifying and removing from the manufacturing environment support infrastructure that doesn’t add value, such as eliminating clean-in-place (CIP) and steam-in-place (SIP) systems, and treating water for injection (WFI) and buffer preparation and supply as services
-
Ensuring that the design conforms to European GMP requirements, genetically modified organism (GMO) requirements, and local environmental constraints
-
Looking at the facility room segregation and sizing to maximize the ability to reconfigure manufacturing process rapidly and ensure availability of space for future requirements
We used a two-pronged approach to rapidly screen and develop design concepts. First, to evaluate the technologies, we applied a risk-based method to assess and screen single-use equipment for performance and supply chain reliability. Second, we used BioSolve Process modeling software from Biopharm Services to quickly evaluate economic and facility impacts of various options.
Using that approach, the team screened about 100 different options and scenarios in a two-week period. We considered factors such as the impact of cell-line MAb expression titer increases over time, variations in different processes, and quantities of material required for clinical supply. Further considerations included how single-use technologies would affect floor area, process flow, support infrastructures, and warehouse storage. The adopted layout was then incorporated into the architectural concept, and we generated a full virtual three-dimensional (3-D) model of the facility before construction. Like all other Pierre Fabre construction projects, ABU was built with a strong emphasis on optimal integration into the landscape, preserving large green spaces, and strong architectural design (Photo 1).
Photo 1:The ABU building is divided into two wings linked by administrative offices, with a total area of 23,700 ft2. Its design maximizes reconfiguration opportunities, providing space for running two products through the facility at a time. That allows for reconfiguration of downstream processing (DSP), offers the capacity to handle titer changes, and provides for on-site preparation of WFI and buffer solutions.
Using single-use technology demands an optimized flow of consumables and solutions. Preparation and warehouse areas must be located as closely as possible to the manufacturing areas. A controlled, nonclassified (ISO 9) material hall serves as a central corridor between the ABU process and preparation rooms. Buffers are prepared in purified water using closed, disposable bags and then moved through the material hall. The bags’ contents are delivered to process equipment through specific tubing transfer hatches. That decreases the amount of clutter within classified areas, simplifies equipment access, allows for clean rooms to be reduced in size, and improves integration of the facility as a whole.
Single-use equipment tends to be more compact than the stainless steel equivalent, which helps reduce the size of production areas. In our facility design, surfaces of ISO 8 and ISO 7 process rooms are 20% smaller than in a traditional facility with equivalent capacity.
Cleanroom volume is also smaller than it would be in a traditional facility. Ceilings are 2.7 m high in all classified areas, except in the upstream processing (USP) room just above the bioreactors, which has a ceiling height of 3.6 m. This represents a 26% decrease in volume compared with the GMP suites in the Pierre Fabre Immunology Centre, in which all ceilings are 3.8 m high to accommodate stainless steel bioreactors. The new design concept also allows for downsized heating, ventilation, and air conditioning (HVAC) systems, with a 33% reduction of air supply requirements for the cleanroom areas.
Single-Use TechnologyEquipment selection for the ABU plant followed a structured approach, using a formal risk assessment and criticality analysis for all components. We created a decision matrix tool to support side-by-side evaluation of similar equipment from several suppliers. The tool incorporated about 30 criteria — including technical aspects, cost analysis, and supplier performance — to help us make informed decisions and select the systems best suited to the company’s needs.
All selected equipment was based on single-use technologies. Not only do they provide greater safety for products and processes, but they also require less physical infrastructure to support manufacturing. As a result, the ABU facility has no steam generation and no CIP systems.
One factor critical to this project’s success was a close partnership with suppliers — in particular with EMD Millipore, which provided most of the DSP equipment. We selected Mobius FlexReady Solutions with Smart Flexware assemblies (Photo 2) for tangential-flow filtration (TFF) and chromatography, despite the fact that they had not yet completed commercialization. The systems offer a unique single-use flow path and additional features:
-
The fully automated system is easy to set up, requiring only a minimum number of components for operation.
-
The flow path is designed for easy installation onto the system, with very few moving parts and low hold-up volume. Assembly inlets and outlets are clearly labeled, and a “clamshell” m
olded flow path provides error-proof guidance. -
The same control platform is used for all chromatography and TFF steps, which reduces operator training, shortens the learning curve, and speeds introduction of each process.
-
The systems were conceived with interchangeable modules that allow for rapid changeover from batch to batch, from unit operation to unit operation, and from one scale to another.
The Mobius FlexReady clarification and viral filtration systems were not sized to the actual and future perspectives of ABU processes. So the Pierre Fabre team designed two clarification skids working in parallel with EMD Millipore to enable operations across wider membrane areas (Photo 3).
Photo 3:The viral filtration skid was also modified to accept larger membranes (Photo 2). And to achieve required performance, we adapted new single-use Quattroflow pumps from PDG Dover to that skid. The team also developed a unique 1,500-L, double-jacketed tank on load cells with a recirculation loop to collect clarified supernatant. This system increases flexibility in ABU downstream operations.
We chose single-use bioreactors from GE Healthcare and Thermo Scientific — similar to those already in place at Pierre Fabre’s pilot facility — to ensure seamless transfer and scale-up (Photo 4). The control system is the same for all bioreactors, which simplifies training and process supervision.
Photo 4:Support Systems: Single-use equipment requires adaptation, which involves rethinking how to connect systems or transfer product through the successive steps of a manufacturing process. We created and/or installed a number of new devices to meet those needs, and they differ notably from what is commonly observed in traditional stainless steel bioproduction sites.
Disposables require numerous tubing connections with built-in connectors. From a regulatory standpoint, this is recognized as an open process step. For the ABU plant, specially designed laminar flows were mounted on height-adjustable mobile devices, equipped with H14 filters, and validated to ensure an ISO 5 environment in the vicinity of each connection.
In addition, transfer hatches were specifically created as a cost-effective solution to accommodate passage of multiple tubes from the material hall to classified areas. The system accommodates a range of tubing diameters and any type of connector. Media and purification buffers are transferred directly to process equipment, simplifying movements of containers through the facility. An air-pressure cascade from high to low classification is maintained with controlled ventilation in each transfer hatch to provide adequate segregation of areas (Photo 5).
Photo 5:We also implemented a Biosafe port between the ISO 8 and ISO 7 DSP rooms. Along with ready-to-use Biosafe bags, the port helps prevent cross-contamination risks and guarantees a closed method of transferring products to final purification. Port holes were also included between areas for which containment is not a key requirement (e.g., between the upstream-processing and harvest rooms). The port is made of stainless steel piping with a PTFE shutter, which allows for passage of single-use tubing (Photo 3).
Finally, all process equipment (such as bioreactors and chromatography skids) is plugged in for pharmaceutical fluids, information technology (IT) networking, and power supply through specific connections located in the ceiling of each classified area. This allows operators to move easily around the equipment and clearly segregates fixed systems from disposable tubing. The material hall and most process areas are equipped with additional utility panels to handle future process adaptations.
Facility FunctionSingle-use technologies offer flexibility but add to requirements for operator manipulation. Ergonomics, simplicity of use, and proper training were key concerns and important criteria during equipment selection. The team worked jointly with suppliers to ensure seamless use of equipment and components. For instance, technicians reported that the filter holder of the Mobius FlexReady virus filtration system was not in an appropriate position. So EMD Millipore made adjustments by adapting the skid’s lateral panels into its doors to facilitate setting and connections of filters.
Many process bags are made with the same design and connectors to allow for interchangeability at different stages of a process. We also collaborated with suppliers on preassembled manifolds to reduce the number of connections, errors, and process resources. In under a week, production operators were trained by EMD Millipore on each unit-operation system, and each trainee was issued a certification for inclusion in the GMP files.
One supervisor and four operators take charge of all raw-material weighing, buffer preparation, and upstream and downstream activities. In this configuration — with approp
riate support for warehouse management, quality control (QC), and quality assurance (QA) — the facility can deliver 10 clinical MAb batches per year (6.5 weeks for each batch). With additional process resources and aggressively overlapping production runs, 15 batches per year are feasible. However, massive and repetitive production is not a primary goal for this facility; the main aim is to adapt quickly to new projects and deliver cost-effective products for clinical trials.
All aspects of routine maintenance were developed with engineering and maintenance teams. Large plenums above the working areas provide direct access to technical equipment and utilities so that supervision, diagnostic decisions, and interventions can occur without engineering or maintenance staff entering classified areas (Photo 6). Equipment simplicity and an absence of CIP and SIP also reduce maintenance and costs.
Photo 6:Over the short term, the ABU plant’s capacity can be rapidly expanded with the addition of a 1,000-L or 2,000-L bioreactor after addition of supportive process equipment. That configuration does not require a facility extension and will be suitable for phase 2 clinical batch production.
Environmentally FriendlyThe ABU facility was built with a clear commitment to achieving an environmentally friendly production facility rating. It is a pioneer in HQE (High Quality Environmental standard) certification, which is the French national reference for the environmental performance of industrial buildings. In fact, the ABU plant has officially been classified as an “experimental operation” to serve as an example of HQE labeling for the industrial sector, including laboratories. Its sustainability efforts save both energy and water while improving water treatment.
Energy Savings: Air-treatment systems are the most energy-demanding components of a bioproduction facility. To reduce energy use, the ABU project team selected systems with high efficiency and superior environmental performance. Each HVAC system is positioned just above the cleanroom related to it. The short ductworks make few turns and are dimensioned for low pressure drop. Those systems are also connected to the building management system (BMS) to allow for temperature-adapted modes during nonactivity periods.
Lowering air-change rates in classified areas during idle periods is currently being investigated. The results should bring further energy savings. In addition, all facades have been inspected using thermography to detect heat loss, and all observed defects were corrected.
Energy requirements for artificial lighting are smaller than for HVAC and heating, typically accounting for 4% of total energy use. Nevertheless, the team addressed it as well by favoring natural lighting in all work spaces and installing low–energy-consumption systems. Occupancy sensors and adjustable lighting were installed in corridors and in the process areas. For the process areas, two levels of light intensity are available: normal and low. The normal intensity is triggered by human activity in an area; the low intensity is used during inactivity and for visual inspection of areas from outside corridors. In addition, the facility design promotes natural light to limit the amount of artificial lighting needed. Corridors on the facades have full-length windows that favor light transmission and provide high performance in solar control and thermal efficiency.
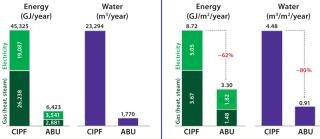
We benchmarked ABU energy use against Pierre Fabre’s existing immunology center facility. The CIPF has a traditional 2 × 600-L stainless-steel fermentation train and several classified areas for GMP batch production, so it was considered a good model for a comparability exercise. Yearly annual energy consumption was 62% lower in the new facility when data were normalized (Figure 1).
Water Savings and Waste Treatment: Working with single-use systems ensures a tremendous reduction in water use. After a year of activity in the ABU facility, water consumption was 80% lower than that of the existing facility (normalized for energy use by square meter of usable surface).
A system was designed to collect and segregate all process liquids in the ABU plant. Effluents with potential GMO contents (bioreactor output, for instance) are stored in a dedicated tank and heat treated. Others effluents are collected in their own tanks and automatically adjusted to neutral pH before elimination in the public sewage network. This facility uses no solvents and very few caustics.
Drought-tolerant lawn grass was planted so that no watering system would be needed. A landscaped ditch at the rear of the building includes a large underground tubing system to collect rainwater. That allows for regulated release of extra water into the municipal storm-drainage network.
Goals AchievedThe company implemented this new facility’s features to allow it to run efficiently while minimizing the environmental footprint and providing a great place to work. Equipment was selected from the most advanced and high-performing bioproduction technologies. Whether off-the-shelf or customized, they allow for fast, safe, simplified batch production and will make it easier to rapidly reconfigure the facility in the future.
This project was delivered on time for GMP production and kept under budget, with overall costs 30% lower than average for the pharmaceutical industry (on a dollar/ft2 basis). The first 1,000-L technical and clinical batch runs behaved exactly as expected, with a perfect overlapping profile of cell culture and purification when compared with 250-L batches from the CIPF pilot plant. The result demonstrated the benefits of cutting-edge facility design, single-use equipment, and innovative support systems that are designed to streamline processes and ensure product quality.
Author Details
Corresponding author Olivier Cochet, PhD, is director of industrial biotechnology (