Three major concerns predominate biotechnology executive management in organizations of all sizes and above all other risks: finance (or its absence at critical moments), technological performance, and failures in coordination. Some business functions, such as human resources (HR), are effectively siloed horizontally and therein are more likely to be susceptible to only one of those risks (1). Few functions are subject to this trinity of risks simultaneously; all functions may be exposed to failures in internal coordination, and a smaller subset can be prey to challenges of both internal and external coordination. But manufacturing can be exposed to all three concerns (2).
Biotechnology companies are vertically integrated by nature – and particularly companies pursuing contemporary novel biological therapeutic platforms such as cell, gene, and viral therapies (3). They operate within a predominantly virtual business model by outsourcing key functions to domain experts such as contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs). Outsourced tasks otherwise would necessitate high operational and capital expenditure but, relative to an overall business, would register low-use levels if brought in-house (4).
Start-up biotechnology companies are likely to spend 50% of their first two years of operating budgets on CMO services, seeking to move academic manufacturing processes to manufacturing levels suitable for producing good manufacturing practice (GMP) material for phase 1bâ2a clinical studies. When translated into crude numbers, that cost usually is between US$2.5 and $6 million (5). By comparison, contract research organization (CRO) services to conduct preclinical good laboratory practice (GLP) studies and to prepare materials for investigational new drug (IND) submissions are likely to cost somewhere between US$750,000 and $1.5 million, with the randomized control trial (RCT) costing US$1 to $5 million alone (6).
The sobering reality is that most start-up biotechnology companies will spend nearly half of their hard-raised capital on CMOs to produce material critical to filing IND applications and conducting preclinical studies (7). Considering the significance of the figures involved, the current vacuum (rather than corpus) of harmonized processes and standards to support evaluation and appointment of such critical vendors seems to be an oversight with potential for significant repercussions. This is the central issue that creation of biopharmaceutical vendor evaluation and selection minimum standards (BioVesel) will seek to resolve.
How the Current Landscape Emerged
The lack of harmonization and standards in CMO tendering processes results from both evolution and inertia, the exact origins of which are unfortunately impossible to pinpoint. However, we see the following factors as contributing to the current status quo.
Specialization of Biopharmaceutical Procurement: Historically, pharmaceutical procurement departments focused on procurement of nonspecialized (and often high-volume) services. The focus was on price rather than technical performance or differentiation (8). Therefore, deep, lasting relationships were forged on a now-flawed and outdated basis between procurement departments of biopharmaceutical companies and key vendors, including CMOs. Conversely, procurement of biopharmaceutical CMO services requires a structured approach centered on ensuring that each technology is treated as de novo given the significant technological diversity of product pipelines and the multiplicity of experience, equipment, and capacity among available CMOs.
Limited Public Sector Participation: Most comparable sectors in terms of procurement value and complexity of service have significant public sector involvement (e.g., aerospace and defense industries) (9). Their procurement processes are consequently subject to public-sector procurement processes and standards. With the exception of early stage public-health applications, the biopharmaceutical industry remains largely independent of public-sector activities.
Dominance of Preexisting Relationships and Contractual Structures: Companies that have negotiated a master service agreement (MSA) or the (dreaded) first statement of work (SoW, also known as SoW 1) with a major vendor (10) often have been surprised by the months taken to finalize the SoW â and the sometimes substantial accompanying legal bills. Such companies will understand the convenience of remaining with an existing vendor for pipeline programs, independent of that vendorâs expertise (or lack thereof) or track record with other specialist technologies (11).
Opacity of CMO Attributes: An initial major challenge in CMO procurement can be simply identifying CMOs at a primary level, then understanding their respective expertise and capacities at a secondary level (12). Awareness of potential CROs for inclusion is based mostly on word-of-mouth, personal experience, conference presentations, and general awareness of larger âblue-chipâ CMOs with significant experience in legacy technologies such as small molecules, fine chemicals, and dyes (13). A single compendium of CRO capacities and expertise does not exist. Therein lies an inclusion bias in CRO selection to tender processes.
Agency Relationships: Biopharmaceutical vendor contracting is subject to an inherent linearity and interconnectivity whereby selected tools and technology manufacturers in turn make introductions to selected CMOs, which then recommend their own preferred CROs (14). This informal network has some merits in (for example) leveraging industry experience. However, it also suffers from a reporting bias due to individual agency relationships and the lack of a compendial reporting resource for all suppliers (15).
Technological Inertia: Most large-scale request-for-proposal (RFP) processes have reverted to centralized tendering software platforms that require vendor and purchaser
registration and prequalification. Such platforms confer some transparency to the tendering processes, but coordinating the requirements of multiple vendors and products would be challenging (16).
Limitations in Vendor Incentives for Progress: Ultimately, established vendors are disincentivized to invest in cross-industry platforms â which would of course include those of competitors â to support tendering activities (17). Service purchasers would benefit from investment in such platforms, but companies with a limited number of assets would have a corresponding limited need to use such platforms frequently, curbing the return on investment (ROI).
Human Capital Constraints Among RFP Specialists: In other industries, entire subsectors have emerged with companies offering RFP support services or project-managing RfP processes. This is now slowly emerging in biotechnology (18) but remains limited in adoption across company sizes and platform technologies for active pharmaceutical ingredients (APIs).
Biopharmaceutical Vendor Evaluation and Selection Minimum Standards (BioVesel)
Standardization of procurement processes leads to resource optimization and best practice. This stands in contrast to ad hoc processes that, as indicated by our collective experience above, may eventually yield outcomes but through an approach that is cumbersome and rarely yields optimal results.
BioVesel intends to develop a standard form of procurement of services through a common industry framework. It will enable new entrants to benefit from a procurement procedure that already is in place. It therefore proposes to achieve this without recourse to developing new processes and will prevent clients from incurring the inefficacies incumbent in nonstandard purchasing and outsourcing. Existing organizations will benefit from contributing to a common marketplace and optimizing procurement services over time.
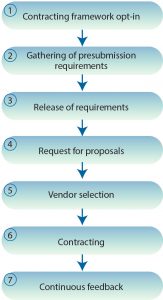
We suggest the following seven steps to establish a basis for procurement of CMO services (Figure 1).
Contracting Framework Opt-In: A not-for-profit organization and/or independent multiple-stakeholder advisory group with membership drawn from the community will be responsible for maintaining BioVesel standard(s) and developing procurement procedures. New entrants will register with this organization and gain access to process and standard commercial templates and legal contracts. Those documents can be modified and extended by member organizations to fit individual circumstances, but they will be sourced from standard agreements maintained by the framework organization.
Gathering of Presubmission Requirements: Requirements for services will be gathered against a standard pre-RFP submission checklist, taking into account factors that influence analysis, design, and initiation of services. Common issues and pitfalls incumbent to requirement gathering will be structured against a standard risksâactionsâissuesâdecisions framework. Detailed interview questions for key stakeholders will allow for the critical capture of data required for development of an RFP.
Release of Requirement: A pre-RFP notification will be sent to all framework members to indicate an associated need. This will allow for preparation of resources necessary for a response. During this stage, the sourcing organization will complete a templated RFP that will be validated by the BioVesel not-for-profit for best fit.
Request for Proposals: An RFP will be released.
Vendor Selection: Vendor selection will be completed against preselected criteria.
Contracting: Following vendor selection, standard framework templates will form the baseline for the commercial offer, subject to any project-specific requirements.
Continuous Feedback: Users of all guidelines and templates will compete a short evaluation questionnaire after use, which will be collated and used by the not-for-profit organization and/or independent multiple-stakeholder advisory group to propose and implement updates.
Next Steps
We will develop a preliminary research and engagement proposal as a basis for stakeholder engagement and discussion. Once that proposal is acceptable to the community following discussion and peer review, we will convene an advisory board to develop the envisaged not-for-profit organization to develop, maintain, and enhance the BioVesel framework and associated standards. This also will include participation from standards implementation organizations including the National Institute of Science and Technology (NIST) and notified bodies such as the British Standards Institution (BSI).
Procurement process for CMO services at first may appear to be a mundane and perhaps âdryâ area for multiple-stakeholder engagement and academic research. However, the adage that âsomeone has to do itâ perhaps best encapsulates our motivation to address this industry-wide need.
It is challenging to be emotive about a potential standard. But in purely commercial terms (notwithstanding the prospect of greater transparency, improved corporate governance, and opportunity to minimize coordination risks), if the standard reduces the lead time to CMO contracting by a single month for an organization, that saves one month of burn and accelerates critical time to market by a month. The benefits will be exponential for a number of stakeholders: for CMOs, that can better plan capacity and resources; for potential CMO clients, that will be able to save time and money and operate in a more transparent environment; and also, ultimately, for patients.
We propose that all interested stakeholders now work together to ensure that substandard procurement practices become a thing of the past. They are not a valid reason to protract unmet patient needs. Now is the time to bring this too-long-neglected horse to predefined selection of a suitably standardized watering hole – where it can thrive in a healthy and sustainable environment.
References
1 Baldi S, Vannoni D. The Impact of Centralization on Pharmaceutical Procurement Prices: The Role of Institutional Quality and Corruption. Regional Studies 51(3) 2017: 426â438.
2 Glass HE, Beaudry DP. Key Factors in CRO Selection. Appl. Clin. Trials 17(4) 2008: 52.
3 Gbadegeshin SA. Stating Best Commercialization Method: An Unanswered Question from Scholars and Practitioners. International Journal of Social, Behavioural, Educational, Economic, Business and Industrial Engineering 11(5) 2017: 1088â1094.
4 Dillen L, Verhaeghe T. Outsourcing Bioanalytical Services at Janssen Research and Development: The Sequel Anno 2017. Bioanalysis 9(15) 2017: 1195â1201.
5 Lowes S. Outsourcing in Bioanalysis: A CRO Perspective. Bioanalysis 9(15) 2017: 1161â1164; doi:10.4155/bio-2017-4994.
6 Hayes R. Bioanalytical Outsourcing: Transitioning from Pharma to CRO. Bioanalysis 9(15) 2017: 1149â1152; https://doi.org/10.4155/bio-2017-4996.
7 Welink J, et al. White Paper on Recent Issues in Bioanalysis: Aren’t BMV Guidance/Guidelines âScientificâ? Part 1 â LCMS: Small Molecules, Peptides and Small Molecule Biomarkers). Bioanalysis 9(22) 2017: 1807â1825; doi:10.4155/bio-2017-4975.
8 Sanderson J, et al. Towards a Framework for Enhancing Procurement and Supply Chain Management Practice in the NHS: Lessons for Managers and Clinicians from a Synthesis of the Theoretical and Empirical Literature. Health Serv. Deliv. Res. 3(18) 2015.
9 Decoin M, Boisleux Charlet C. A Portrait of the Ideal CRO. Phytoma La Defense des Vegetaux 1997; www.phytoma-ldv.com/article-22651-Phytoma_La_Defense_des_ vegetaux_et_l_8217_evolution_de_la_ protection_des_cultures.
10 Ferrario A, et al. Challenges and Opportunities in Improving Access to Medicines Through Efficient Public Procurement in WHO European Region. WHO Regional Office for Europe (Copenhagen, Denmark, 2016); www.euro.who.int/en/publications/abstracts/challenges-and-opportunities-in-improving-access-to-medicines-through-efficient-public-procurement-in-the-who-european-region-2016.
11 Spooner N, Cape S, Summerfield S. Outsourcing Strategies in Bioanalysis. Bioanalysis 9(15) 2017: 1125â1126; https://doi.org/10.4155/bio-2017-4986.
12 Cohen B, Neubert M. Price-Setting Strategies for Product Innovations in the Medtech Industry. In EuroMed Research Instituteâs 10th Annual Conference of the EuroMed Academy of Business, Rome, Italy, 13â15 September 2017.
13 Handfield R. Patient-focused Network Integration in Biopharma: Strategic Imperatives for the Years Ahead. CRC Press: Boca Raton, FL, 2016.
14 Danzon PM, Mulcahy AW, Towse AK. Pharmaceutical Pricing in Emerging Markets: Effects of Income, Competition, and Procurement. Health Economics 24(2) 2015: 238â252.
15 Abberley L. Outsourcing of Bioanalysis at GSK: A Hybrid Approach with a Robust Support Model. Bioanalysis 9(15) 2017: 1139â1144; https://doi.org/10.4155/bio-2017-0062.
16 Craddock A, Nadarajah S. Future Trends in Outsourcing: A Summary of the Bioanalysis Zone Survey. Bioanalysis 9(15) 2017: 1127â1129; doi:10.4155/bio-2017-4985.
17 Slack D. eProposals: An Interactive Approach to eProcurement. Appl. Clin. Trials. 6, 2009; www.appliedclinicaltrialsonline.com/eproposals-interactive-approach-eprocurement.
18 Grohn K, et al. Lean Start-Up: A Case Study in the Establishment of Affordable Laboratory Infrastructure and Emerging Biotechnology Business Models. J. Commercial Biotechnol. 21(2) 2015.
Alison R. Carter is a research fellow, Edward Meinert is a Sir David Cooksey research fellow, and David A. Brindley is a senior research fellow in Healthcare Translation and Group Lead at the University of Oxford, Healthcare Translation Research Group, Department of Paediatrics, Oxford, UK; corresponding authors: david.brindley@paediatrics.ox.ac.uk and alison.carter@paediatrics.ox.ac.uk.