Single-use technologies have been implemented in biomanufacturing facilities all over the world. Inherently more flexible than stainless steel equipment, single-use technology allows for more rapid technology transfer by minimizing the time it takes to design, purchase, and qualify new capital assets. Rapid turnover between batches is facilitated with no need for protracted clean-in-place (CIP) and steam-in-place (SIP) regimes; the risk of product cross contaminations is reduced because single-use fluid-contact surfaces are never previously exposed to a biopharmaceutical product stream. For those reasons and more, single-use technologies have been widely adopted within the industry, especially by leading contract manufacturing organizations (CMOs).
Different levels of automation are available for those implementing single-use solutions, from completely manual steps through semiautomated to completely automated unit operations. The industry seems to a have a preference for semiautomation, in which a degree of manual intervention remains possible (1). The desired level of automation will of course depend on the type of operation. Complex operations such as perfusion cell cultures can run for many days or weeks. Multiple process streams must be controlled simultaneously, which almost certainly requires a high level of automation. The case for the full automation of buffer filtration, on the other hand, is not as straightforward because it is a much less complex operation. In addition to operational complexity, the cost of implementation and then ownership needs to be considered when deciding on the level of automation required.
Here we present a case study describing reasons for automation of a final aseptic filtration and container filling step within a CMO facility, with the decision-making process the company used to determine an appropriate level of automation.
Final Product Manufacturing
Fujifilm Diosynth Biotechnologies is a CMO with locations in Research Triangle Park, NC, and Billingham, UK. Together they offer over 25 years of combined experience in development and manufacture of recombinant proteins, vaccines, and monoclonal antibodies expressed by a wide array of microbial, mammalian, and insect cell systems.
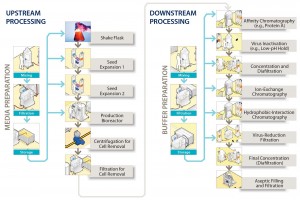
Bulk filtration and filling into containers is the final step in such manufacturing processes before products are transported to the location where they will be put into vials. Figure 1 illustrates the location of the step at the end of the downstream process, showing a generic process-flow diagram for a biopharmaceutical manufacturing process. At this stage, considerable value already has been added to products based on the cost of consumables and operations required to make and purify them. Few unit operations remain that could remove contaminants introduced during this step before the final filling of a product into vials. Even though this bulk-filling operation is not frequently discussed at conferences or in literature, it is nevertheless of considerable importance.
Final bulk filtration and container filling at Fujifilm Diosynth Biotechnologies’ UK manufacturing site has been performed using different methods according to the facility and the type of molecule being filled. An open filtration and filling method had been contained within a vertical laminar flow (VLF) cabinet. A closed method also had been used, particularly for filling high-potency biologics for which preventing product cross-contamination is critical. Performing the step with high-potency molecules inside the VLF cabinet was made even more undesirable by the difficulty of validating its complete cleaning because such molecules can be active in extremely small quantities. Both the open and closed fill methods make use of single-use technology and are highly manual processes.
The inherent flexibility of single-use technology allows for a wide range of process configurations. It is frequently perceived as an enabling aspect of disposables because a consumable fluid path can be readily designed around a given process. This greater flexibility, however, also brings challenges. For example, ensuring the compliant operation of new processes can become more difficult with single-use technology. When Fujifilm Diosynth Biotechnologies was designing new mammalian cell culture manufacturing capacity at its UK site, a decision needed to be made regarding the best approach to adopt for final bulk filtration and filling in the new facility.
Biologics CMOs are expected to develop, transfer, and manufacture according to good manufacturing practices (GMPs) “right the first time.” Achieving that objective is a major undertaking that requires considerable planning, management, and experience. Some people might consider final bulk filtration and container filling of purified biopharmaceuticals to be a relatively simple task compared with, for example, a chromatography step. But the considerable coordination of people, materials, and methods involved generates operational complexities at a point in the process when the value of the product is at its greatest. Photo 1 gives an impression of the complexity of this step.

Photo 1: Elements that must be orchestrated for a right–first-time, GMP-compliant, drug-product
bulk filtration and dispensing step include the product itself; process specifications and
manufacturing batch records; trained operators, quality assurance personnel, and one quality control representative; a qualified laminar flow cabinet, consumables, and labels.
Product Arrives in the Bulk-Filling Suite: The first point of uncertainty here is the precise time that a product arrives in the final filling suite. Days, weeks, or even months may have elapsed since the manufacturing process was initiated by removal of a vial of cells from freezer storage. Given that timing predictions are based on a limited number of small-scale runs, all intervening operations from the working cell bank through to final bulk filtration will have a degree of uncertainty around that parameter. Although CMOs tend to prefer clients’ processes to move swiftly through their facilities (to maximize the number of slots they can sell each year) most will recognize that can be achieved only through dependable operations performed on time and right–first-time.
Defined Process with Associated Manufacturing Batch Record: Once a product arrives in the bulk-filling suite, it can be processed only if a number of other conditions are met at the same time. For example, a developed and specified processing method is required along with an approved manufacturing batch record. Processes in early development stages may be operated with a predefined level of flexibility to manage manufacturing uncertainties.
Personnel: A final bulk filtration and dispensing operation requires trained operators with experience in the skilled competencies needed to perform the task. If multiple platforms are used across a site, scheduling those operators into the suite when the product becomes available can pose a challenge. This unit operation requires a minimum of three people in the suite at once to execute, witness, and document the methods involved. In addition to production operators, representatives from quality assurance (QA) must be present to verify the operation. And a single representative from quality control (QC) also must be available for dynamic environmental monitoring while the step is under way.
Supply Chain Coordination: Single-use consumables must have been delivered, received, released, dispensed, transferred, tracked, assembled, sterilized, and configured in the suite before the product arrives. Consumables may be sourced from multiple suppliers and need to have been assembled before use. Obtaining such materials in time for the bulk filling to take place on schedule will have required months of planning on the part of the supply chain team. Their task may have been made more difficult by uncertainties about the process design, which might be finalized at an extremely late stage.
Equipment Availability: Before a VLF cabinet is used, it must be cleaned, assembled, serviced, calibrated, and requalified for each operation of the process.
Approved and Released Labels: Finally — and not insignificantly — labeling for the bulk-product filled containers must be approved and released by the quality unit so that they can be tracked and ready for use as the containers are filled. Labels must be reconciled upon completion of the filling stage.
Clearly this “simple” step actually involves many degrees of complexity. We wondered whether it could be simplified.
Collaborative Work

Photo 2: Automated SciFlex Filter and Dispense
single-use system for product bulk filtration and
dispensing
Fujifilm Diosynth Biotechnologies and Parker domnick hunter collaborated to develop an automated final bulk filtration and container filling system (Photo 2). The fluid flow-path is completely single-use, with manifolds designed for closed-system processing. Using this automation, over 100 L of bulk purified biopharmaceutical product can be filtered and filled into either bioprocess containers or bottles with volumes from 100 mL to 2 L and more. Filling accuracy for each bottle or container is ±10% of the target fill volume. The system can allow for manual filtrations with constant pressure and flow rate or Parker domnick hunter’s patented R/P Stat hybrid method that maximizes filtration capacity.
Following filtration but before container filling, an automated, in situ filter-integrity test is performed. In the unlikely event of an integrity test failure, the system allows for reprocessing by repeating the filtration procedure. This system works with any commercially available filter capsule. A human– machine interface (HMI) allows operators to access intuitive software that is easy to learn and use. Because of the high-value product at this part of the manufacturing process, the system is designed to recover nearly 100% of the product volume.
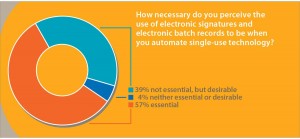
Electronic Records and Signatures: Mention the word automation to engineers within the biopharmaceutical industry, and many will make word associations with phrases such as electronic batch records and electronic signatures (ERES). In a recent poll, we asked 75 participants this question: “How necessary do you perceive the use of electronic signatures and electronic batch records to be when automating single-use technology?” Over half (57%) of respondents said that ERES were “essential,” and over a third (39%) replied that although ERES were not essential, they were desirable (Figure 2). Only 4% of respondents described ERES as being “neither essential nor desirable.”
In our opinion, whether ERES are required depends on the context even within GMP manufacturing. Use of ERES is applicable mostly to processes in which the only feasible solution is to collect data electronically:
- When the type, amount, and complexity of data required to demonstrate compliance exceeds what could be captured readily on paper batch records (e.g., chromatograms from chromatography steps), or
- When the number of operations that must be monitored and controlled to achieve compliance exceeds what could be captured readily on paper batch records (e.g., maintaining control within a fermentor).
Although ERES could have been built into the automation, the system we designed operates without them at the request of Fujifilm Diosynth Biotechnologies. The CMO chose instead to employ an automated systems engineer who could ensure that the system would be compliant with good automated manufacturing practice (2) because the filtration and dispensing operation did not fit into either of the above scenarios. Primary records for the final fill operation at Fujifilm Diosynth Biotechnologies are paper-based batch records and automatically printed product labels; the final fill system stores no electronic records of product dispensing weights.
System access is restricted, with different levels of privileges available. Instructions for each process are retained within the batch records. The system is not networked, but it can print method parameters for attachment to documentation. However, those can be printed only when the operation is being performed.

Photo 3: A barcode interlocking mechanism
ensures that the automation will run only after
the correct manifold has been installed.
For regulatory compliance, the system has a number of interlocking mechanisms that ensure its correct operation. First, physical guidelines are located on the system in such a way that manifolds cannot be installed incorrectly. Stepwise diagrams on the HMI guide operators through a correct installation procedure. Finally, each manifold has a unique bar-code identifier that the system must read before the automation can work (Photo 3). The latter feature offers additional benefits such as an ability to track manifolds through accounting software, thus increasing traceability and assisting with fault finding if that is necessary.
Benefits Realized
The role automation can play in minimizing process variation and increasing the consistency with which operations are performed to improve product quality is described elsewhere (1). Automation is one strategy for reducing manual errors.
Published frameworks illustrate how errors can arise for different reasons depending on each operational situation and the level of conscious involvement of operators performing a given task (3). With new operational systems or those used infrequently (e.g., right–first-time manufacture of a biologic), work is carried out in a “knowledge-based” mode: Operators are likely to carry out such tasks with full consciousness (4). But an operator then can become overwhelmed with information, increasing the risk of mistakes being made.
| Closed-System Automation Project Benefits Eliminates the need for vertical laminarflow cabinets Provides a system for filling potent molecules Provides a step change in safety and quality compliance Offers built-in processing flexibility while retaining a consistent operator experience Reduces the number of operators required to perform filling step Reduces batch-to-batch variation Standardizes operations, leading to • A larger operator pool trained to perform filling step (site standard across five streams of manufacturing) • Shorter process development and document generation times • A simpler supply chain with reduced component part numbers |
Acknowledging those challenges, Fujifilm Diosynth Biotechnologies worked with Parker domnick hunter to reap the benefits of automation. In addition to reducing variation from manual intervention, we believe that the benefits of installing this automated filtration and filling technology will increase the CMO’s operational flexibility through improvements in standardization and employee cross-training. The box above summarizes the project benefits.
Adoption of an automated system for final bulk filtration and filling will provide teams at the UK site with a standard template for this unit operation that can be used for multiple processes. Our intention is to standardize bulk filtration and filling on site such that, regardless of the individual facility in which a given operation is performed, the same method will be used. That should maximize familiarity and facilitate technology transfer between facilities within the company’s manufacturing organization.
Many of the complicated steps that operators execute require considerable skill but have been automated. Reduced operating complexity will allow for fewer operators in the suite, further improving the environment for GMP operations by minimizing human sources of contamination. In addition, the new system will standardize the expertise required by each operator. Closed processing means that the VLF cabinet will not be required, so associated scheduling of cleaning, assembly, and qualification can be eliminated.
Automating this step will reduce complexities associated with the bulk filtration and filling operation, so risks to compliant manufacturing of new products also will be reduced. Development teams will have a “target in their sights” to aim for. This will help them more efficiently generate timely process descriptions and documentation.
A degree of flexibility has been built into the system design to allow for inevitable process changes. For example, the volume to be filtered can increase, fill containers can be added or taken away, and the volume of those containers can change. Even so, the overall standardization of the operation will reduce the number of component part numbers that supply chain teams have to source, thus helping them plan further into the future than they could before.
Finally, the system automatically prints labels for documentation and filled containers, reducing the reliance on quality teams to deliver and track those into the suites. Clearly, the effective scheduling of this step will be much enhanced.
Operational Flexibility
This case study has illustrated many benefits that can be realized from automating single-use operations. Those benefits extend far beyond freeing up operator time or reducing manual errors. Automating final bulk filtration and container filling at Fujifilm Diosynth Biotechnologies in the United Kingdom will facilitate technology transfer and right–first-time, GMP-compliant manufacturing through significantly improved operational flexibility.
References
1 Hutchinson N. Understanding and Controlling Sources of Process Variation: What Represents the Greatest Risk to Achieving Product Critical Quality Attributes? BioProcess Int. 12(9) 2014: 24–29.
2 GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems. International Society for Pharmaceutical Engineering: Tampa, FL, February 2008.
3 Rasmussen J. The Role of Error in Organizing Behaviour. Ergonomics 33(10) 1990: 1185–1199.
4 Reason J. Human Error. Cambridge University Press: Cambridge, UK, 1990. •
Dr. Paul P. Bird is head of the manufacturing engineering group at FUJIFILM Diosynth Biotechnologies, Belasis Avenue, Billingham, TS23 1LH, UK; 44-1642-363511. Dr. Nick Hutchinson is global market development manager at Parker domnick hunter, nick.hutchinson@parker.com.