A number of recent improvements in the engineering of high-titer expression vectors, in biopharmaceutical process development, and in facility construction have converged to present new opportunities for cost-effective, flexible, biomanufacturing facility construction. The evolution of requirements for biopharmaceutical facilities is driven by globalization of the biopharmaceutical industry, patent expirations of several blockbuster biopharmaceutical products, and the increasing shift in new product development away from blockbuster drugs and toward more personalized, niche products.
An increase in product approvals (primarily monoclonal antibodies, MAbs) and sales growth of 10% per year for the past five years have transformed the biopharmaceutical industry almost exclusively into a “monoclonal antibody industry.” MAb-related products now represent a significant portion of all biopharmaceuticals approved to date and are anticipated to continue to drive future demand for biopharmaceutical manufacturing capacity (1,2,3). Further, as many early MAb products come off patent, the competition to develop and market biosimilar versions of those products is rapidly increasing (4).
Not only are biosimilar sales expected to grow in the US and European markets, but increasing demand for access to affordable biologic products in the emerging markets of Brazil, Russia, India, and China (BRIC) will also stimulate further growth in such sales. Coupled with a desire for local production of critical medicines, the anticipated sales growth will lead to increasing demand for manufacturing facilities that can be installed and operated within those countries. So the need is clear for relatively simple but flexible biomanufacturing facilities that can be easily replicated in multiple locations.
Like all other biopharmaceutical products, MAbs traditionally have been manufactured in large facilities with multiple fixed, stainless steel bioreactors ranging in size from 100 L to 20,000 L; fixed and inflexible downstream processing space; and complex piping for delivery of buffers and media, product transport, and cleaning of the large number of fixed stainless steel tanks and other equipment. Such facilities were oftendesigned to produce a single product each or for campaigning just a few products. But a number of trends are converging to create a demand for smaller, more flexible, and cost-effective manufacturing facility options. Those trends include dramatic increases in product titers and yield, advancements and availability of single-use technologies, pressure to reduce health-care costs, a desire (or in some cases requirement) for local production, and increasing focus on personalized medicine for small niche markets.
Increasing Product Titers: One of the most significant trends in biomanufacturing over the past decade has been a dramatic increase in volumetric productivity for processes based on mammalian cell culture, particularly MAb expression. Through development of improved, engineered parental cell lines and expression vectors, improved media and feed compositions, and a more robust ability to measure and control bioreactor process conditions, several groups have reported antibody titers of 10 g/L or higher in cell culture supernatants (5,6,7,8). Combined with improvements in downstream processing, overall process yields of 1–3 g/L are now common.
Although such product titers and yields are not yet routine, more and more companies are achieving those levels of productivity. So it is reasonable to expect that most new MAb manufacturing processes soon will routinely reach productivities of >5 g/L. High product titers coupled with downstream process yields of ≥70% (9) will result in overall process yields of >3 g/L for many MAb products in development — and even for those already on the market after next generation processes are implemented.
With those productivities, a 15,000-L bioreactor will produce 75 kg of drug substance per batch, and a traditional “six-pack” facility (incorporating six 15,000-L bioreactors) could produce a metric ton (1,000 kg) of product in just six weeks. The largest currently marketed cell culture–derived products require about one metric ton per year. So this is a significant advancement over previous levels of productivity and suggests that future biomanufacturing facilities will be built around fewer and smaller bioreactors (e.g., two or three 2,000-L to 5,000-L reactors). Although the bioreactor scale will be reduced, those facilities will yet require significant downstream processing operations to support purification of their bioreactor output.
Single-Use Technologies for Biomanufacturing
Over the past decade, single-use process technologies have been increasingly incorporated into the manufacture of biopharmaceutical products. Applications range from Lonza’s use of GE Healthcare’s Wave bioreactors in the inoculum train for large-scale production bioreactors in its Portsmouth, NH facility (10) to Shire’s reliance on single-use bioreactors and other disposable technologies in the upstream portion of its new ATLAS commercial manufacturing facility (11, 12). Companies are now considering facility designs that incorporate disposables at all stages of manufacturing (13, 14).
Many forces are driving adoption of such technologies in biomanufacturing, particularly for multiproduct facilities. Disposables increase flexibility, reduce requirements for expensive critical utilities (e.g., water for injection, WFI, and clean steam), decrease requirements for equipment cleaning and cleaning validation, lower capital investments, and shorten facility construction times (15, 16).
Although those reasons for implementing single-use technologies in biomanufacturing are compelling, some challenges and concerns related to their use remain. Disposables do increase ongoing operating costs associated with the purchase and disposal of consumables. Leachable and extractable substances from their product-contact surfaces may contaminate products. Sourcing limitations are associated with purchases of single-use equipment, for which no consistent and uniform stan
dards yet cover the numerous connectors required for installation and use. And there is a current lack of regulatory experience with many such technologies.
Nevertheless, biopharmaceutical manufacturers — ranging from small start-up companies to large product sponsors and CMOs — are increasingly implementing disposables. Single-use bioreactors are especially popular in manufacturing biopharmaceuticals both for clinical trials and commercial sale.
Single-use bioreactors currently come in volumes up to 2,000 L and have been used to produce clinical-trial material at 20-L to 2,000-L scales. The earliest such technology made widely available for use in bioprocess applications was the Wave Bioreactor system (now from GE Healthcare Life Sciences in Uppsala, Sweden), a self-contained disposable bag with agitation/mixing provided by an external platform rocker (17). After that early attempt at a disposable bioreactor, several companies developed alternatives that resemble the standard, cylindrical, stirred-tank design that is already prevalent in the biopharmaceutical industry’s installed base of stainless steel bioreactors. Examples include Xcellerex (now part of GE Healthcare Life Sciences), Thermo Scientific HyClone, and Sartorius Stedim Biotech. For their single-use bioreactors, a fixed support containing process control software is installed in a production facility, then the disposable bioreactor is provided as a bag that can be inserted into that support structure (18). Agitation is generally provided by an installed impeller that is disposable like the bag and attaches to a motor in the support structure. Feeding, pH, and dissolved oxygen (DO) are controlled through installed ports and probes.
Several studies show that cell culture processes and the critical quality attributes of products made by such processes are comparable in single-use and stainless steel bioreactors of the same size (19,20,21). For example, Smelko, et al. demonstrated that for high-intensityChinese hamster ovary (CHO) or NS0 cell culture processes, product from single-use bioreactors in standard configuration up to the 1,000-L scale is comparable by all biochemical analyses to that from a 1,000-L stainless steel bioreactor (22). In that study, the seed train, harvest, and all downstream processing steps were performed using identical equipment and methods, with the type of bioreactor being the only difference.
Photo 1:
Photo 2:
Photo 3:
Other disposable bioreactor systems have been developed with new geometries and mechanisms of cell agitation, mixing, and control of nutrients and pH. Marketed bioreactors with square or rectangular shapes from companies such as ATMI Life Sciences have been shown to support mammalian cell growth with mixing characteristics and productivities similar to those of more conventional cylindrical geometries (23). PBS Biotech has introduced another design using an Air-Wheel mixer driven by sparging gases (24). An orbital shaker with ≤1,500-L production volumes was also introduced recently (25). When the unit is operated at suitable speeds, its geometry provides adequate mixing and aeration without an impeller. Like the single-use bioreactors of more traditional design, these alternative-geometry types also consist of a fixed support shell in which a disposable bag is fitted for use.
For single-use bioreactors to realize their full potential in biomanufacturing, both users and suppliers have identified a number of areas and opportunities for improvement. They’ve pointed to the need for better disposable sensor elements, particularly those that can maintain calibration over the extended run times of today’s high-performance cell culture processes (26). Other potential improvements include larger transfer ports to facilitate harvesting and large-volume transfers and improved construction of disposable bags to reduce leakage failure rates.
That last issue is of great concern to companies implementing single-use bioreactors because bag leaks can result in costly losses during production runs. To help address this problem, ATMI recently introduced a new test system as part of its release criteria for disposable bags to confirm their integrity before they are shipped to customers (27). The test method involves flooding a fully assembled single-use bioreactor with helium and noting any escape of the gas with specialized sensors mounted on the exterior of the bag. ATMI claims that this method can detect holes as small as 10 ÎĽm, making the technique much more sensitive than other bag leakage tests.
All those and many other innovations in development are certain to continue improving single-use bioreactors and facilitate their implementation in the future.
Single-Use Technologies for Downstream Processing: Development of single-use downstream processing products for effective and economical operation at clinical or commercial-manufacturing scales has lagged behind that of disposable bioreactors. But recent significant efforts on the part of both established and new suppliers of bioprocess equipment and separations products have brought about a wide range of scalable, single-use products for downstream processing.
For clarification, multiple vendors now offer disposable depth filters at multiple scales. So biomanufacturers can choose between multiuse stainless steel housings for conventional depth filters or single-use depth filter cassettes with disposable product-contact surfaces for clarification operations. Many commonly used depth-filter media from
different suppliers are now available in both conventional and single-use formats (28, 29).
Similarly, the first single-use cassettes for cross-flow filtration applications became available in the past decade and are now widely used in biomanufacturing. In addition, “single-pass ultrafiltration” systems are in development. They allow for tangential-flow concentration and diafiltration without recirculating the retentate solution. The approach could be converted to a disposable format in the future.
One of the most challenging unit operations to convert to single-use technologies has been chromatography, which is the heart of most biopharmaceutical purification processes. Several suppliers now offer prepacked chromatography columns in plastic or low-cost glass housings.
They are designed to be discarded after one or more cycles, but because of the high cost of chromatography media, some manufacturers choose to use them for a campaign of several batches rather than disposing of a column after one run.
Although that provides a workable solution up to a certain scale, some practical limitations may prevent the use of disposable chromatography columns at very large scales. To circumvent those limitations, several companies are developing continuous chromatography technologies such as simulated moving bed (SMB) to reduce the scale and capital cost of chromatography columns and systems needed for downstream processing. At least one company, Tarpon Biosystems, is establishing SMB with a fully disposable flow path (30). As with other continuous processes, SMB is inherently more scale-efficient. So more material can be purified with such technology using current-scale disposable chromatography columns than would be possible using conventional batch operations.
As an alternative to column chromatography, membrane adsorbers are already widely used for flow-through applications, in which a product of interest flows through while impurities remain bound to the column. A common application is the use of anion-exchange membrane adsorbers for clearance of nucleic acids or host-cell proteins in MAb processes (31). Unfortunately, current membrane adsorbers lack the capacity of column media for chromatography steps in which the product binds to the medium (so-called “capture” steps). So the industry needs new membrane adsorber technology that provides robust, scalable, high-capacity membrane adsorbers before these devices can be used widely in commercial-scale biopharmaceutical “capture” applications.
The use of disposable bioreactors and downstream processing products will continue to increase well into the coming decade, becoming routine for production of both clinical trial materials and commercial products. In a recent analysis of the economic aspects of single-use systems for biopharmaceutical and vaccine manufacturing, one conference presenter concluded in 2010 that although they are more economical for manufacturing at production scales roughly <8,000 L, above that level conventional stainless steel systems become economically more attractive (32). Given that and the high efficiency of modern biomanufacturing processes, future facilities will predominantly incorporate multiple single-use bioreactors of 2,000 L or smaller (13). For those few products that require larger production volumes, commercial manufacturing using traditional stainless steel bioreactors will continue, especially as a way to use the existing large installed base of such manufacturing capacity.
Modular Biopharmaceutical Manufacturing Facilities
In a recent discussion of next-generation manufacturing facilities, an author argued that biomanufacturing facilities can be divided into process, facility, and infrastructure components (33). Each plays a significant role in the success of a manufacturing enterprise. A failure or weakness in any one will lead to poor product quality and/or inefficient manufacturing. Improvements in manufacturing technologies and advancements in single use systems have clearly transformed bioprocesses. Hand in hand with those process improvements comes modular construction, which has been around for decades. It will become more and more common because modular alternatives can have smaller footprints than traditional facilities and be built rapidly in locations where cleanroom and piping expertise may not be readily available.
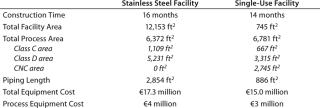
Together, modular technology and single-use technologies can reduce investment and operating costs as well as potential financial risk for new biopharmaceutical manufacturing facilities (34). Table 1 describes an example of the economic benefits that could come from combining the previous-generation modular facility construction combined with disposables. As we will show in a future article, the current generation of modular construction and design technology (along with further advances in single-use technologies) could lower capital and operating costs even further while enabling construction of comparable facilities in under 12 months.
Table 1: Estimated facility costs for a 1,000-L bioreactor facility
In Table 1’s preliminary study, the use of disposables reduced labor costs by about 30%, although raw materials costs were almost 20% higher for the single-use facility. The result is a net savings for each manufacturing campaign of about 10%. Additional savings in operating costs are also achieved through elimination of column packing and elastomer change-outs as well as lowered validation, calibration, and equipment preparation requirements. Overall batch processing time is shorter in the single-use facility, further improving process economics through an increased number of batches that could be produced there.
As the example shows, modular construction offers advantages such as improved quality, increased flexibility, and (perhaps most important) more cost-effective construction. Modular construction generally requires a more modest up-front capital investmentthan do traditional facilities. Each module making up the final facility can be fabricated at a company that is highly familiar with the unique requirements of biopharmaceutical facility construction. And much of the fixed equipment can be installed and tested at that manufacturing site before the fully assembled modules are shipped to an intended manufacturing site. That minimizes technical and regulatory challenges that come with using traditional building strategies to construct an entire facility on site. A modular facility can be moved easily if necessary after its initial installation — or readily expanded as needed.
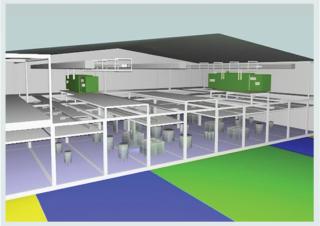
Photos 4:

<div
style=’float:left;margin-left:0px;margin-top:5px;margin-right:0px;margin-bottom:7px;background-color: #f7f7f7;width:auto;’>
Looking Back: Modularization is a type of outsourcing that involves moving some construction work from an actual facility location to a different site, whether domestic or international. Varying degrees of modularization always have been common in biopharmaceutical facility design. For example, many biomanufacturing unit operations involve process skids, on which equipment is mounted and moved into and out of a production cleanroom area. In addition, equipment modules are used to divide up the work load or reduce potentially invasive construction during retrofits. Both strategies allow complex, process-related equipment to be constructed in a controlled environment before being installed in an actual facility.
In addition, construction and installation of complete modular production facilities for pharmaceuticals and biopharmaceuticals has been widely accepted. More than 70 such modular facilities have been built worldwide since 1986. One pioneer in such construction was Pharmadule, a Swedish company that is now out of business. Many of the assets and key personnel of that company, however, are now part of KeyPlants in Stockholm, Sweden. Other companies that offer modular facility construction include Biologics Modular of Brownsburg, IN (www.biologicsmodular.com) and G-Con of College Station, TX (www.gconbio.com).
Some traditional bioprocess equipment suppliers such as GE Healthcare Life Sciences, Sartorius Stedim Biotech (SSB), NNE-Pharmaplan, and Merck Millipore have begun to offer fully equipped modular facilities. Through its Enterprise Solutions group, GE provides fully assembled, qualified, and ready-to-run KUBio module facilities equipped with GE equipment. Similarly, SSB offers a G-Con FlexMoSys modular cleanroom facility equipped with SSB single-use systems and standardized hardware.
They are attractive and appropriate for simple installations, but such prepackaged modular facilities have some limitations. The KUBio system, for example, comes only in a stand-alone facility design and uses GE process equipment exclusively, which limits flexibility and choice in design and precludes its installation within an existing building shell. A FlexMoSys facility, on the other hand, comes in both indoor and outdoor versions, so it can be used either as a stand-alone facility or installed inside an existing shell. The G-Con system does not use SSB equipment exclusively, so users do have some choice in process equipment. However, the current FlexMoSys design does not accommodate bioreactors >1,000 L. Another drawback to all prepackaged modular facilities currently available is that they are designed for bulk manufacturing only, without accommodations for fill–finish operations.
Looking Forward: KeyPlants has developed an innovative approach to modular facility design and construction that is flexible and cost efficient, and it allows for the use of process equipment from all suppliers. Compared with the above options, the flexibility of this design allows installation of any size bioreactors from any vendor and provides greater flexibility in the layout and design of both upstream and downstream process areas. Modules can be installed and operated within an existing building or as a separate modular building as long as suitable power sources are available.
Additionally, the KeyPlants design can incorporate both bulk drug manufacturing and fill–finish operations into a single plant design.
Generally, the more efficient project execution resulting from modular design and construction can significantly reduce time to market, which increases the net present value (NPV) and return on investment (RoI) for a new facility. In a future article, we will compare and contrast the costs and economic implications of modular and traditional facility designs in more detail. Companies should consider the time and risk factors involved in facility construction as well as more obvious factors such as capital and engineering costs. When exploring those economics, it is important to include such variable costs such as that of architectural, engineering, and construction management services; interest rates on applied capital; and risk of delays in facility construction. Those variables are common in traditional construction but less so in modular facilities. The latter approach provides the best capital efficiency by ensuring that investments are made only in the minimal-scope options.
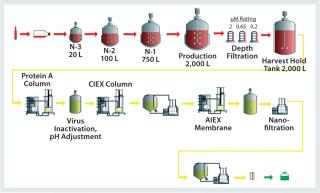
The key to modular design is its integration with the manufacturing process and division of that process into key functional modules that can be replicated throughout a facility, which allows for standardization. KeyPlants has prepared such a standard design for a MAb manufacturing facility based on a typical platform process (Figure 3). The process design is based on a 2,000-L single-use bioreactor, with a typical inoculum train and downstream process (35). It further incorporates disposable prepacked chromatography columns; single-use membrane adsorbers; bags for buffers, media, and product intermediate storage; and maximum use of disposable filters and other process components. To minimize the cost of the prepacked columns — especially for the Protein A affinity chromatography step — column recycling is incorporated for each manufacturing batch.
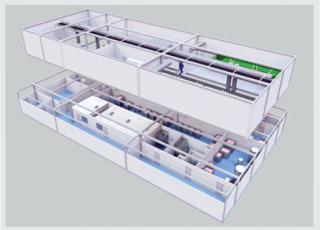
Photo 5:
Using that standard platform process as a basis for design, prefabrication and fabrication of the individual process modules is simplified and variability is minimized in construction time and project execution. For modular construction using such a standardized design, 75–80% of the total construction process takes place at the modular facility company’s factory, with the remaining 20–25% of construction activities being performed at a final facility site, which greatly reduces site congestion while increasing safety. Consequently, the overall modular-construction approach significantly mitigates the inherent risk of construction, installation, and validation.
One primary difference in today’s modular facilities compared with previous approaches is that they include all necessary functionality and equ
ipment required for a biopharmaceutical facility. The modular facility can be delivered as a free-standing building (façade and roofing included) or as indoor modules for installation into an existing building or prefabricated shell. A modular building (whether indoor or outdoor) comprises prefabricated structural modules. Their structural design meets relevant codes (for the European Union, United States, and others), including earthquake requirements if required.
To ensure full compliance with global regulatory standards and expectations, the floors of individual modules are made of reinforced concrete or metal sheet plates with PVC or epoxy coatings. Cleanrooms for product processing activities are constructed using a panel system, with integrated walls, ceilings, doors, windows, and so on. The cleanroom design includes walkable ceilings with adequate space for air-handling equipment, ductwork and piping, and electrical distribution, as well as service access and maintenance from above. The same design is used in isolated rooms for electrical supply, plant utilities, and clean utilities. By locating all support systems (electrical; piping; heating, ventilation, and air conditioning, HVAC; and so on) in easily accessible space above the cleanrooms, each prefabricated module will integrate distribution of such systems, with only one hook-up required at the facility site. This ensures a true “plug-and-play” design and minimal installation work.
Sustainability: One key advantage to modular buildings is that they typically include features that qualify them for LEED (Leadership in Energy and Environmental Design) certification (36). Leveraging factory build processes and more efficient supply lines, a modular facility can take significantly fewer resources to construct and deploy than a site-constructed, purpose-built facility would.
Incorporating single-use process technologies into a modular biotech facility could further add to its benefits. Rawlings and Pora have shown that a facility based on single-use technology is about 50% less energy intensive than one based on stainless steel systems because the consumption and heating of large volumes of water to clean and sterilize reusable equipment is more energy demanding than producing and disposing of plastic bags that can also be incinerated for energy recovery (37). Hodge has estimated that incorporating single-use technologies in a modern facility can result in an about 85% reduction in water usage and waste compared to a traditional stainless steel facility (38). However, the savings resulting from this reduction in water usage and waste is partially offset by the cost of disposing the approximately three-fold increase in solid waste, primarily plastic from the disposable bags and bioreactors, generated by the single-use facility.
Studies by Mauter (39) and Pietrzykowski, et al. (40) have recently shown that the overall impact of single-use technologies on waste flow — and environmental impact — are minimized over the entire life-cycle of such technologies. For example, Pietrzykowski, et al. showed that over the full life cycle of production, the cumulative energy demand (CED) and global warming potential (GWP) for MAb production in single-use systems is 34% and 32% of CED and GWP for a stainless steel facility, respectively (40).
Taking into account all of that and the markedly increased yields of modern, high-titer processes, it becomes evident that a next-generation facility incorporating single-use technologies will have a significantly smaller carbon footprint per kilogram of monoclonal antibody produced than would a traditional manufacturing facility using stainless steel systems.
Author Details
Corresponding author Howard L. Levine is founder, president, and principal consultant of BioProcess Technology Consultants, Inc., as well as founder and chief operating officer of Biocrescentia LLC (12 Gill Street, Suite 5450, Woburn, MA 01801-1728; 1-781-281-2701; hlevine@bptc.com). Jan Lilja is commercial director of KeyPlants AB; Rick Stock is a consultant for BioProcess Technology Consultants, Inc.; Hans Hummel is business development director at KeyPlants AB; and Susan Dana Jones is vice president and senior consultant of BioProcess Technology Consultants, Inc.
REFERENCES
2-S46.