Autologous chondrocyte transplantation is a modern experimental therapy for treatment of degenerative intervertebral disc diseases. Several studies with animal models have shown that transplantation of cultivated autologous chondrocytes can delay progression of disc degeneration. A few products based on human autologous chondrocytes are already on the market. Repair of disc damages with grafted chondrocytes appears feasible in the near future. So chances are growing for clinical applications meant for restoration of original disc function. As a result, optimization and standardization of the individual steps of the laborious therapeutic approach gain even more importance.
Apart from minimally invasive treatments for removal of chondrocytes and later retransplantation, the laboratory process requires a long cultivation period under in vitro conditions. Successful proliferation of each patient’s own chondrocytes is the main focus. Ideally suited for this approach is a standardized cell culture with highly reproducible conditions. Common practice is cultivation under normoxic conditions because it is easier and provides cells with constant conditions when medium is changed and during transport through ambient air.
PRODUCT FOCUS: AUTOLOGOUS CELL THERAPY PRODUCTS
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: QA/QC, PROCESS DEVELOPMENT, AND MANUFACTURING
KEYWORDS: CELL CULTURE, PAT, QUALITY CONTROL, MEDIUM CHANGE, OXYGEN AND PH MONITORING
LEVEL: INTERMEDIATE
It must be considered, however, that oxygen concentration within a disc nucleus is very low under in vivo conditions. Because chondrocytes are used to such conditions, transferring them into a normoxic culture environment actually could be harmful. Sensitive processes of in vitro cell proliferation and differentiation can be achieved routinely only by strict adherence to defined culture parameters. Patient-specific therapies should thus provide significantly higher success rates than more general products.
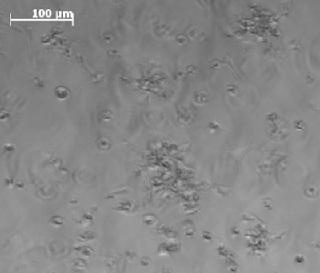
Growth Control in 3D Culture SystemsHydrogel-based three-dimensional (3D) cell culture systems offer particular advantages for tissue-engineering in cell-based therapies for disc degeneration (1,2,3). Chondrocytes grow notably better within a gel matrix rather than in a monolayer culture because such a spatial environment better resembles the situation within an intervertebral disc. The only disadvantage of such systems is that — by contrast with thin monolayer cultures — light-microscopic control is impossible for both culture quality and morphological differentiation within a dense matrix (Figure 1). Judging the quality of 3D culture systems thus requires consideration of alternative parameters.
We have analyzed and evaluated the availability of oxygen within culture medium and kinetics of the pH value for the first time. Both parameters are considered key factors for successful and reliable in vitro cultivation and differentiation. Accurate measurements of oxygen consumption during cultivation help cell culturists reach conclusions regarding the physiological state and quality of their culture conditions.
Noninvasive Detection of Oxygen and pH Value with the SensorDish Reader: Measuring both culture parameters has been impossible to date for continuous, precise monitoring in cell culture — mainly due to a lack of miniaturized and sterile measurement techniques. Introduction of the SensorDish Reader (SDR) device by PreSens Precision Sensing GmbH (www.presens.de) has closed the gap.
The system consists of two components (Figure 2): a 24-channel luminescence lifetime reader and 24-well multidishes. The latter are equipped with oxygen- or pH-sensitive optical sensors, respectively (OxoDish brand for measuring oxygen and HydroDish brand for pH). This optosensoric measuring system enables noninvasive detection of both culture parameters close to the cells. It allows simple and reliable detection of set-point deviations throughout a culture process. This leads to clearly improved safety for cultivating sensitive cell lines as well as a fast, standardized culture process with time- and cost-saving advantages.
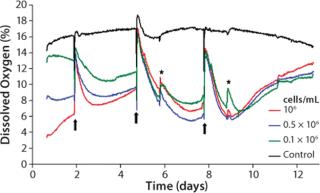
We characterized the kinetics of dissolved oxygen (DO) and pH value with a well-established 3D culture method using chondrocytes of calf-tail nucleus pulposus (3, 4). Three defined cell densities of 0.1, 0.5, and 1.0 × 106cells/mL were cultured on a proprietary hydrogel matrix for about two weeks in OxoDishes HydroDishes, respectively. Each well contained 2 mL of buffered culture medium at pH 7.4. During the study, we changed the medium three times (on days 2, 5, and 8) in both cell samples and controls. Online monitoring began shortly after seeding the cells. Optical control measurements were performed on days 6 and 9. For this purpose, the multiwell dishes were removed for a short time from the standard incubator atmosphere of 5% CO2 and 95% relative humidity. We obtained oxygen kinetics by calculating the average of three replicates per cell density. Cell-free cultures served as a control.
Oxygen Kinetics As an Indicator for Culture Medium Change: Throughout the cultivation process, samples with identical cell density are characterized by steady and reproducible oxygen kinetics. Our averaged values allow for direct comparison of the oxygen kinetics for each of the three studied cell densities (Figure 3). In the first cultivation phase, the cells adapt to their new environment in the 3D matrix and redifferentiate to functional chondrocytes. As expected, the highest-density samples reveal the greatest oxygen consumption and the lowest culture media oxygen content at the beginning of measurement. At medium exchange, DO from the air-saturated medium increases the oxygen level. Depending on the starting cell concentration, the oxygen content of the samples decreases again after medium exchange due to proliferation and synthesis of new matrix under high metabolic activity. Lack of substrate and possible inhibitory effects decrease cell activity.
The kinetics correlate closely with the initial cell densities: When cultivation begins, the decline of DO is fastest. A loss of activity occurs earliest for the sample with the highest cell density. At the same time, passive oxygen diffusion from the incubator atmosphere into the medium causes a continuous oxygen increase until the next medium change. More medium changes at a point when cells are more active presumably reveal additional growth and shorten the cultivation time.
After the second medium change, oxygen decreases faster and to lower values, correlating with the maximum of matrix synthesis after about seven days (5, 6). Again, decreasing activity is seen for reasons described above. During the entire cultivation, the activity of samples with different starting cell concentrations resemble each other more and more. This indicates that the proliferation of cells with the highest starting cell concentration gets lower, whereas those cells with lowest starting concentration increases, until a maximum cell density is obtained and the first layer of new chondrocyte matrix is built. Synthesis of new matrix is then lowered to a minimum, reflected by an oxygen minimum after the third medium change, which is similar to that after the second change. A steady state in matrix synthesis is associated with decreased cell metabolism, which causes a more rapid oxygen increase.
Within the controls, the oxygen content is mostly stable at 16–18% DO. Close to the end of cultivation (after day 9), a slight decrease in DO is evident. It can presumably be ascribed to changes in density within the hydrogel matrix after a long-term culture process.
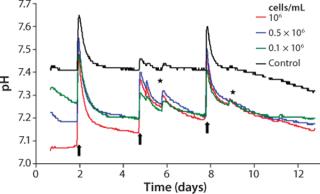
Culture Quality and pH ValueLike oxygen measurements, pH kinetics reveal characteristic dynamics according to the length of cultivation and initial cell density (Figure 4). The kinetics among samples with identical cell densities are very close. The pH values decrease depending on their starting cell concentration, however, without falling beyond pH 7.0. At higher initial cell densities of 1.0 × 106 cells/mL and 0.5 × 106 cells/mL, the respective pH minima are reached within 24 hours. At the lowest starting cell density of 0.1 × 106 cells/mL, a minimum pH is not reached before the first medium change. Like the DO kinetics, pH kinetics also show the equalization of cell densities at the end of cultivation despite the different starting cell concentrations.
Apart from changes of culture medium and effects of opening the incubator door, the controls display a largely stable pH value. Analogous to DO determination and the reasons mentioned there, pH values of the controls start decreasing slightly after day 9. Because of the change of culture medium, a brief pH shift beyond the physiologically optimal initial value takes place for both samples with cells and cell-free controls. When nonpreincubated medium is used, this can lead to a pH value shift of about +0.2.
The reason for that increase and the subsequent sharp decrease of pH kinetics originates in the cell culture medium’s buffer system. The pH value of the latter is adjusted indirectly by means of the incubator’s CO2 atmosphere. Media that have not been preincubated in such an atmosphere reveal, as a consequence, enhanced pH values until they are equilibrated again with the incubator atmosphere. Equilibration can last up to a day because gas exchange happens only through passive diffusion. A similar effect is also detected when the incubator door is opened, even for short periods, when CO2 leaks out to almost completely. Consequently, the pH value of the culture medium inside increases.
Within the cell-containing samples, the sharp decrease in pH kinetics after medium exchange precedes a slower downward trend. After the first culture medium change, the pH of those samples with the highest cell concentration declines the most. The other two kinetics display a similar trend but with a less prominent descent. Similar to the oxygen measurements, the pH value decreases faster after the second medium change. The reason for that is the continuous cell proliferation and the associated higher metabolic activity.
Superimposing effects of a steep rise and fall of pH after medium change and the metabolically determined downward trend impede an exact interpretation of observed pH kinetics. Analogous to oxygen measurements, a less pronounced downward trend points to a diminished metabolism caused by lack of substrate. But a precise statement on culture quality and the optimal date of medium change cannot be given based on these findings alone. Through continuous pH detection, however, cell culturists can prevent pH decreases to adverse low values caused by enhanced metabolic activity, which can inhibit cellular activity or even cause cell damage. Online monitoring of pH thus provides valuable hints regarding when changes of culture medium are necessary.
Quality ControlNoninvasive monitoring of DO and pH with the SDR is well suited for in-process assessments of culture quality and determination of the optimum time for medium change in 3D cell cultures. Culture processes can be monitored even more exactly and reliably in the future. It is likely that using the culture system described here enhances the quality of autologous chondrocytes for transplantation. Through DO kinetics, standard protocols for an even more efficient cultivation of chondrocytes may be derived, and the optimum time for chondrocyte harvest determined. Furthermore, the SDR enables for the first time a precise insight into 3D cell cultures by detecting available oxygen.
Precise measurements and the advantage of continuous, sterile data acquisition underline the high potential and advantage for future applications of this system in tissue engineering. Besides monitoring metabolic substrate acidification, the pH measuring system is also an exceptional instrument for protection and control of buffer parameters in a given culture medium. Without interruption, the high resolution of the system indicates all systemic fluctuations of an incubator’s atmosphere — e.g., by manual interventions such as door openings or medium changes. Using such instrumentation, companies can enhance the quality of cell and tissue products and sooner realize their therapeutic benefit to patients.