With an increasing number of cell therapies becoming available for patient use, the need for controlled and consistent manufacturing and delivery of cell products is increasingly important. A closed cell culture process not only offers control and consistency, but may also relieve labor demands. Single-use components within a closed process also can reduce contamination risk.
Closed systems with single-use platforms may reduce the risk of biological contamination and cross-contamination that could inadvertently be introduced into cell-culture processes. Such contaminants use culture nutrients to produce unwanted proteins and limit the growth of (or destroy) a cell culture. Slow-growing adventitious agents can be subtle and may become apparent only when unwanted proteins are detected. Detection of contamination will result in unusable product, and depending on the circumstances, entire lots may have to be discarded. This can be incredibly costly in both materials and time.
Manual cell culture processes can be labor intensive and include numerous open events. They have limited process control, which can lead to difficulties in achieving high reproducibility. Some systems are closed during the stages in which cells are grown. It is equally important, however, to close cell seeding and harvested processes. That is more difficult to achieve, so not as many equipment vendors currently address those additional needs.
A Closed Cell-Expansion SystemThe Quantum cell-expansion system offers a functionally closed system intended for use in cell seeding, through feeding and waste removal, and all the way to harvest. This functionally closed, automated, hollow-fiber bioreactor system is designed to grow both adherent and suspension cells. The bioreactor includes a synthetic hollow-fiber bioreactor that is part of a sterile, closed-loop circuit for media and gas exchange. Both bioreactor and fluid circuit form a single-use disposable set mounted onto the Quantum system unit. The bioreactor features ~10,000 hollow fibers with a total intracapillary (IC) surface area of ~2.1 m2. Typical culture manipulations (e.g., cell seeding, media exchanges, trypsinization, and cell harvest) are managed by the computer-controlled system using pumps and automated valves. Those valves direct fluid through the disposable set and exchange gas with the culture medium.
The functionally closed nature of this disposable set is maintained through a sterile docking of bags used for all fluids. Bag connections and disconnections all use sterile-connection technology. Gas control in the system is managed by a hollow-fiber oxygenator (gas-transfer module). Gas is supplied from a user-provided premixed gas tank. By choosing a tank with a desired gas mixture, a user can expand cells at their optimal gas concentration. The IC membrane of the bioreactor is coated with an adhesion promoter to allow the attachment of adherent cell populations.
The Quantum system fluid circuit is designed around two fluid loops — one each for the IC and extracapillary (EC) sides of the bioreactor. Five different lines allow connection of fluid bags to the system for cell loading, cell media support and feeding, waste removal, and reagent addition. They are named the cell inlet line, the reagent line, the IC media line, the wash line, and the EC media line. As a practical matter, the content of the five bags is completely at user discretion. A user may add fluid from any selected bag(s) to the system at a selected flow rate and volume. The system’s flexibility allows fluid to be added to the IC loop simultaneously with the EC loop or independently. The bioreactor membrane between the IC and EC spaces allows free-gas diffusion between the IC and EC sides. It also allows small-molecule diffusion so that glucose and lactate freely pass from one side of the membrane to the other. Larger macromolecules are sequestered on the side of the membrane in which they are added. For that reason, it is important to ensure that media with large molecules critical for cell culture (e.g., cytokines and growth factors) are loaded on the IC side when cells are expanded on the IC side of the bioreactor. The volume of the IC fluid circuit is 189 mL, and the volume of the EC fluid circuit is 305 mL. Fluid may exit the system to either a waste bag or a harvest bag. Because this system has a constant volume, all addition of fluid will result in an equal volume of fluid leaving the system. With the exception of cell harvest, the system always operates with an open fluid path to the waste bag.
A graphical user interface makes operation straightforward. The Quantum system can perform operations ranging from a manual mode (custom task) to full automation of a specific process (automated task). The system’s touch-screen is used to select and perform such tasks. The screen displays buttons to enter data, command the system, and perform a specific action. It also displays real-time information about a system’s condition. Different functions operate the system’s pumps and valves to perform a specified task. In all cases, a user may accept the default values or use the touch-screen to input different parameters.
An “automated tasks” option allows a user to select and begin a task without manually entering a task setting (if the choice is to use the factory default settings for a selected task). Task categories include set management, system management, washout, load and attach, feed and add, release and harvest, and custom. Automated tasks are flexibile: A user can enter task settings manually for an automated task. Also a user can input values for each step of a task before beginning a task. This creates a seamless transition from one step to another.
Valves are managed by the Quantum system’s software, and it will allow the choice of only certain inlet and/or outlet lines according to the task to be performed. For example, the software allows the cell inlet line to be a source of liquid only for the IC side during cell loading. For custom tasks, a user must manually enter all task settings; the system has no default task settings. Users can save custom tasks so that settings do not again have to be entered manually.
Quality TestsAlthough producing cells in a controlled, reliable, and consistent way is of great importance, researchers have understandably raised questions regarding the quality of cells grown in systems without flasks. Because a bioreactor surface differs from the plastic used in flasks, numerous assays have been performed on mesenchymal stem cells (MSCs) grown in the Quantum system to evaluate their quality.
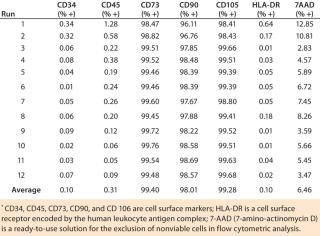
Our flow cytometry results have been consistent among all runs, and positive biomarkers are well within accepted percentages for MSC positives of each biomarker. Results were within acceptance ranges for MSCs as defined in a previous ISCT position paper (1). The biomarkers CD73, CD90, and CD105 should be expressed in MSCs and have high percent-positives because they are a requirement for MSCs (Table 1). All runs were ≥99% positive for those biomarkers. The biomarkers CD34, CD45, and HLA-DR should not be expressed in MSCs and have low percent-positives, all well within the accepted percentages for MSCs. All ru
ns were <2% positive for these biomarkers.
Table 1: Flow cytometry of MSC markers for 12 precultured mesenchymal stem cell expansion runs on the Quantum cell expansion system
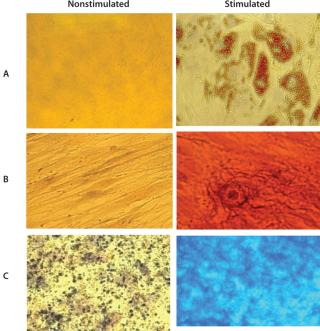
Cells grown in the Quantum system’s bioreactor achieved an average viability of 94% with 7AAD flow cytometry. The experiment demonstrated MSC trilineage differentiation potential into osteoblasts, adipocytes, and chondroblasts for all 12 precultured MSC expansion runs. Those assays use oil red O to stain for adipocytes, alizarin red S to stain for osteocytes, and alcian blue to stain for chondrocytes. Figure 1 shows a representative set of micrographs (400×).
Independent organizations have also conducted tests to evaluate the quality of stem cells in the Quantum system (data was presented at the yearly ISSCR meeting in 2010). Researchers performed functional evaluations of bone-marrow–derived stromal cells (BMSC) by testing bone and myelosupportive stromal formation in vivo in immune compromised mice. Results showed comparable and abundant quantities of bone with adjoining host hematopoietic cells in cohorts of mice that were implanted with BMSC from the Quantum system and cell factories. Researchers concluded that growth in the Quantum system does not alter the differential potential of the subset of stem cells within the BMSC population.
A current Quantum system user is successfully manufacturing a good manufacturing practice (GMP) compliant cell product according to US Food and Drug Administration (FDA) guidelines. This user has noticed a reduced risk of errors and reduced labor. The company had previously been using conventional cell culture methods to produce its cell product and expressed a need to grow significant amounts of adherent stem cells in a consistent, high-quality, and cost-effective method. Using a Quantum system gives this company the potential to run multiple cell-expansion processes simultaneously because all key steps in the process are automated. It also offers the possibility to adjust procedures and save custom tasks. The system also offers them a scalable process as the company continues to grow. This company uses less than one-fourth of the work hours than it did with its former manual process. The user anticipates saving hundreds of hours over the course of the next year.
A Closed-System SolutionA closed, automated technology with a single-use platform for culturing cells, such as the Quantum system, may offer an ideal solution for researchers or companies wanting to ex vivo expand to therapeutic doses of adherent cell types without compromising cell quality. The Quantum system is CE marked under the European Medical Device Directive 93/42/EEC and is listed with the FDA as a medical device.
Author Details
Rachel Kilian is the global product manager for the Quantum system at Terumo BCT;