Evaluating a virus filter should, in theory, be a straightforward exercise. Membrane-based filtration is a robust virus reduction technology that plays an important role in virus safety for most drug production processes. An appropriate virus filter for a given process is generally selected through preliminary testing with relevant drug feed material. Data acquired during such tests are used to determine hydraulic performance targets such as expected flow rates and total throughputs. A virus clearance evaluation study is then performed in which virus is added (“spiked”) into process fluid. Scaled-down studies sometimes referred to as virus validation measure the capability of miniature filtration devices to remove spiked virus. PRODUCT FOCUS: PROTEINS, ANTIBODIES, PARENTERAL PRODUCTS PROCESS FOCUS: DOWNSTREAM PROCESSING WHO SHOULD READ: PROCESS DEVELOPMENT AND MANUFACTURING KEYWORDS: FILTRATION, CONTAMINATION CONTROL, VIRAL CLEARANCE, VIRUS SPIKING LEVEL: INTERMEDIATE
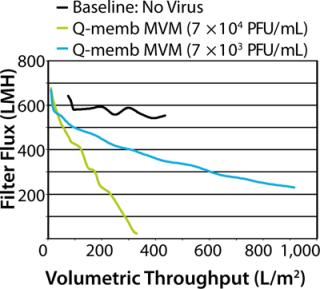
Ideally, during a scaled-down study, adding virus to a drug process solution does not perturb filtration, and a spiked process fluid flows across a filter at the same rate (or pressure) as does unspiked material. Unfortunately, in reality the virus spike frequently has detrimental effects on filter hydraulic performance, resulting in decreased fluid flow. Studies suggest that such filter membrane fouling is generally caused by impurities present in virus stocks rather than virus particles themselves (1,2,3,4,5,6). Spike-induced fouling may prevent achievement of throughput targets, resulting in studies being deemed unsuccessful even though virus reduction was satisfactory. Moreover, the presence of undefined impurities in a virus spike casts doubt on the integrity of a scaled-down model. The purpose of a clearance study is to accurately represent manufacturing, and this goal is subverted when virus spike impurities cause fouling that is not representative of the full-scale process. Here, we recount a virus clearance study in which challenges arose because of unpredicted hydraulic consequences of virus spiking. Fortunately, despite initial difficulties, throughput and virus removal targets were ultimately met thanks to implementation of a flexible spiking strategy. This success was made possible by cooperation among the drug producer, filter manufacturer, and contract testing laboratory specialists. We subsequently reflected upon the challenges encountered in an effort to understand and prevent them in the future. MVM PREPARATION METHODS Crude: 324K cells are infected with MVM. After several days, culture supernatants are collected and clarified. Q-Membrane Chromatography: Crude preparation is passed through an anion-exchange membrane adsorber, which binds the virus. Virus is eluted using protein-free, high-salt buffer. Ultracentrifugation: Virus in culture supernatant is pelleted by high-speed centrifugation. Supernatant is discarded and pellet resuspended in protein-free media. Ultrapure: Virus is grown under optimized low-protein, high-titer-producing conditions. Clarified lysates are concentrated and buffer is exchanged by ultrafiltration. Virus is then purified by ultracentrifugation and flow-through chromatography. This process is patent pending. A follow-up collaborative study examined the root causes of spike-induced fouling and determined how they could have been prevented. We characterized our virus preparations in depth to reveal their protein content and correlate levels to spiking performance. In addition to looking back, we took a step forward using virus purified by an advanced process, which not only solved the problem of premature filter fouling, but also enabled spiking to much higher virus concentrations. Virus spiking has caused uncertainty in virus filtration studies, but improved characterization and purification of virus stocks could make unwelcome spiking surprises a thing of the past. The Dilution Solution Genzyme, EMD Millipore, and Lancaster Laboratories process development engineers and scientists collaborated to conduct a virus-filter clearance study to prepare for upcoming clinical trials with a new monoclonal antibody (MAb G). One regulatory expectation of early stage drug process development is that virus filters should be tested for their ability to remove parvovirus from drug process feed. Parvoviruses represent a worst-case scenario for virus filters, being among the smallest of viruses at ~20 nm in diameter. Minute virus of mice (MVM) is generally regarded to be the most relevant parvovirus for virus clearance of monoclonal antibody feeds generated on murine cells such as Chinese hamster ovary (CHO) cell lines, having previously been identified in a bioreactor contamination event (7,8). Therefore, we used MV M in our MAb G virus clearance study. The MAb G production process was developed using Millipore’s high-flux Viresolve Pro parvovirus filter. Process development data indicated that a volumetric throughput target of at least 1,000 L/m2 could be readily obtained using unspiked material. We set a relatively conservative throughput target for the clearance study at 900 L/m2, and the target log reduction value (LRV) for MV M >4 (virus reduction to less than 10–4 of the original titer). Two types of MVM stocks were available for use as spikes in the study: purified either by Q-membrane chromatography or by ultracentrifugation as described in the “MVM Preparation Methods” box. We selected Q-membrane purified MVM based on scoping trials that indicated good hydraulic performance of retrovirus prepared by this method. MAb G process feed material containing 8 g/L of protein was prepared four days in advance of the clearance study and shipped cold in full bottles to minimize aeration. Immediately before testing, we prefiltered the feed using Millipore MilliStak+ A1HC depth filtration media. Processing unspiked feed across Viresolve Pro devices established a performance baseline (Figure 1). This material performed as expected, with little decline in rate of fluid flow (flux) over the course of the device runs. Baseline throughput was 436 L/m2 at 21% flow decay (flux decreased 21% from the initial buffer flow rate, driven by constant pressure). However, when feed material was spiked with MVM to a target titer of ~7 Ă— 104 PFU/mL, all challenged devices showed dramatic decreases in flux, reaching a final throughput of only 351 L/m2 at 98% flow decay. The implication was that something in the virus-spike preparation had caused the filter membranes to foul prematurely. Consequently, the throughput target was not met, and the success of the clearance study was in jeopardy.
We regrouped to consider modifying the study with a design that would allow us to achieve throughput and LRV targets using the limited amount of remaining feed material. The best option was to reduce the spike concentration in hopes of mitigating the impact of the virus preparation. We reran the test using 10-fold less virus, spiking the feed to a titer of 7 × 103 PFU/mL. The less aggressive spike improved the hydraulic performance considerably, and the target was met with a throughput of 934 L/m2 at 67% flow decay. The spike reduction was not without cost, however. To achieve the LRV target using the more diluted virus spike, a much greater volume of filtrate sample had to be screened for virus, which significantly increased the virus assay expenses. Fortunately, the virus removal was as effective as expected, and no virus was detected in the filtrate, resulting in a final LRV >4.5. The virus clearance study was ultimately successful, but not without causing some anxiety. Although our targets were met, an important question remained: Could the spiking difficulties have been predicted and prevented? We wanted to understand the root of the problem and learn how to improve the situation for future clearance studies. Thus, we conducted a retrospective study to explore the causes of filter membrane fouling upon virus spiking and compare the performance of virus stocks prepared by different methods. Performance Prediction in Retrospect The virus clearance study described above highlights that virus preparations used for spiking can be major sources of uncertainty and complications. Virus stocks are prepared in cell culture and consequently contain cellular and media debris that is not removed during virus purification. Such impurities may decrease the throughput of virus filters, and high-flux devices are especially susceptible (1,2,3,4,5,6). Spike purity is a long-standing problem and a focus of the recently published PDA Technical Report #47: Preparation of Virus Spikes Used for Virus Clearance Studies (9). A major recommendation of this report is that virus stocks used for virus filtration studies should be purified and well-characterized. That was the guiding principle for our retrospective study designed to elucidate factors that contribute to spike-induced fouling and that could have predicted performance in the original runs. This study tested the hydraulic performance of various types of MVM preparations and correlated those data with biochemical attributes of the virus preparations. Lancaster Laboratories used two common methods for purifying its MVM virus stocks: Q-membrane bind/elute chromatography and ultracentrifugation (see “MVM preparation Methods” box). Protein and DNA analyses of the virus preparations showed that both purification methods were quite effective at separating the majority of protein impurities from the virus when compared with the “crude” unpurified source material (Table 1).Table 1: Protein and DNA analysis of MVM preparations
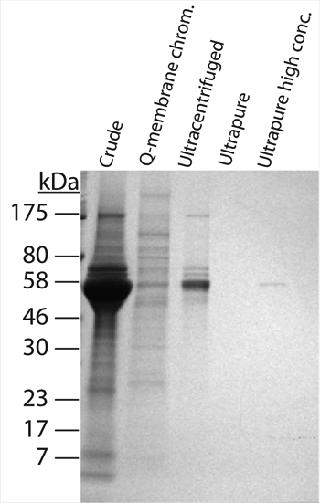
The “fouling potential” of a virus stock can be estimated by the amount of protein or DNA per unit of infectious virus (i.e., pg protein or DNA per PFU) (2). These values normalize impurity levels across stocks that have differing virus titers. They quantify the amount of stock-derived protein or DNA that accompanies each infectious unit of virus in a spike. The MVM preparations contained 100- to 1,000-fold less DNA than protein by weight, suggesting that protein plays the dominant role in fouling. The crude MVM preparation had a relatively heavy protein load, containing 429 pg of protein for every infectious unit of virus (PFU). Q-membrane chromatography and ultracentrifugation reduced those protein impurities to 17 and 13 pg/PFU, respectively. Although total protein amount was similar for both purified preparations, SDS-PAGE analysis revealed that their protein populations were qualitatively quite different. The Q-membrane preparation contained small amounts of many different proteins, and the ultracentrifuged preparation comprised fewer individual proteins in greater amounts (Figure 2). These results suggest that both the quantity and character of proteins in a virus preparation should be assessed to construct a complete protein profile.
We performed filtration runs using MAb G and Viresolve Pro devices to evaluate how virus spike performance correlated with the impurity profile. MAb G process material for these experiments contained 9 g/L of protein and was from a different lot than the previous clearance study. The Viresolve Pro device lot was the same, and the feed material was prepared in the same way using Millistak+ A1HC depth filtration media on the day of testing. We attributed the variability we observed in filtration performance of the unspiked baseline material to volatility in the feed material itself. It may have been sensitive to mechanical handling and changes in aeration as containers were depleted. Filtration device variation did not appear to be a factor because replicate devices yielded consistent results. To account for the run-to- run variability, we always ran replicate baselines in parallel with each experimental group, which allowed reliable comparison of the relative performance of unspiked and spiked material. A series of experiments tested the hydraulic impact of crude, Q-membrane purified, and ultracentrifuge purified MVM preparations. We spiked MAb G feed with each virus preparation to ~ 5 × 104 PFU/mL and processed across Viresolve Pro micro devices. Virus quantification of each spiked feed indicated that the final feed titers for each group were equivalent within the precision of the titration assay (about ±0.5 log). In one representative experiment, the unspiked MAb G baseline throughput was 363 L/m2 at 71% flow decay (Figure 3).
 07b.jpg”/>
07b.jpg”/>
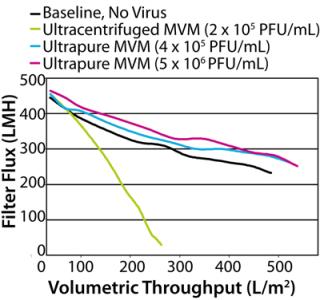
When the process feed was spiked with crude MVM, flux dramatically decreased, and throughput reduced to only 191 L/m2 at 96% flow decay. This was not unexpected, given the high protein content of the crude preparation. More surprising was the profound negative impact of the Q-membrane purified spike. Although protein content was 20-fold lower than the crude preparation, this purified preparation reduced throughput to 238 L/m2 at 91% flow decay, making its performance nearly as poor as that of the crude virus. We checked the Q-membrane spike for virus monodispersity by passing it across a 0.22-µm filter, which would be expected to capture MVM aggregates. That produced no significant loss in virus titer, indicating that virus aggregates were probably not responsible for the accelerated filter fouling. The hydraulic performance of the ultracentrifuged spike was much better. Despite containing approximately the same total protein load as the Q-membrane purified spike, the ultracentrifuged spike had no detectable hydraulic impact, with a throughput of 344 L/m2 at 77% flow decay (similar to the unspiked baseline). One interpretation of these data is that the diversity of protein impurities in the virus spikes — rather than the total amount of protein — was the differentiating factor for membrane fouling impact. The 5 × 104 PFU/mL Q-membrane spike added only 0.0008 mg/mL protein to a feed that already contained 9 mg/mL of MAb, yet it dramatically accelerated fouling of the filter. On the other hand, filtration was not affected by the ultracentrifuged spike, which contributed an equivalent amount of protein. As revealed by SDS-PAGE analysis of the preparations (Figure 2), the Q-membrane purified virus stock contained significant quantities of many proteins. Moreover, the data are consistent with the idea that one or more of those proteins interacted with components of the MAb feed and/or the filter membrane to cause accelerated fouling. Purifying virus spikes using ultracentrifugation may have removed enough of those putative reactive proteins to enable spiking to 4 × 104 PF U/mL without hydraulic impact. It is also possible that the Q-membrane–purified preparation contained other undetected substances (such as lipids) that played a role in filter fouling. In this case, the diverse protein population observed in the Q-membrane preparation may be indicative of generally less effective impurity removal by this purification method compared with ultracentrifugation. The virus preparation protein characterization data would certainly have aided selection of virus stock to use for the original clearance study. In hindsight, the ultracentrifuged MVM preparation, with its cleaner impurity profile, is likely to have enabled achievement of throughput and LRV targets on the initial attempt. That conclusion is supported by the demonstrated hydraulic performance of this preparation in the follow-up study. Nevertheless, even the ultracentrifuged MVM stock contained far more cell culture debris than infectious virus, and it could not be guaranteed that these impurities would not cause issues when spiked into a different process feed or used at a higher concentration. Therefore, we conducted further experiments to determine whether more highly purified MVM could provide a more robust solution to spike-induced fouling. Purity Test Although characterization of impurities can inform selection of virus stocks, even the best conventional preparations may have significant limitations with respect to LRV. Higher LRV claims are nearly always desirable because they provide greater assurance of virus safety. Virus clearance studies using Viresolve Pro devices frequently result in no detectable virus in the filtrate. In such cases, the LRV that can be claimed is determined solely by the titer of virus in the spiked feed and volume of filtrate assayed for virus. Testing volumes are often limited by cost or practicality, so the preferable method of maximizing an LRV claim is to spike with the most virus possible. The upper ceilings for virus spike concentrations are ultimately limited by plugging filter membrane pores with viral particles themselves (5). Ideally, MVM stock titers would be high enough and protein impurities low enough that feeds could be spiked to that limit. However, in many available virus preparations, impurities far outweigh infectious virus, leaving the virus a minority component of each spike. Consequently, spike levels must be kept low enough to prevent impurities from dominating the hydraulic results, especially when using high-flux, small-pore virus filters. Furthermore, virus stock titers frequently are not high enough to enable spiking to the particle limit even if the preparation were sufficiently pure. Ultracentrifuged MVM was the best performing conventional virus preparation, but even this contained mostly nonviral proteins, as shown by SDS-PAGE analysis. Further experiments using increasing amounts of this preparation showed that at higher spike concentrations it caused filter fouling, which limited our ability to raise LRV by increasing the spike titer. To confront the spiking limitations imposed by preparation impurities, we compared an “ultrapure” MVM stock produced at Millipore with the conventional preps (see “MVM Preparation Methods” box). Characterization of the ultrapure preparation showed that the protein content of each infectious unit was extremely low at 0.02 pg/PFU (Table 1). That was attributed to both the very low absolute protein content and the high stock titer. Furthermore, unlike conventional MV M preparations we analyzed, the predominant component of the ultrapure preparation appeared to be virus particles. SDS-PAGE with silver staining and mass spectrophotometric analysis indicated that the preparation contained only two prominent proteins: the major MVM capsid proteins V P2 (64 KDa) and V P1 (83 KDa) (data not shown) (10,11). We compared hydraulic performance of the ultrapure virus with that of the conventional ultracentrifuged preparation in further experiments using MAb G and Viresolve Pro devices. The ultrapure prep enabled high-concentration spiking without detrimental hydraulic impact (Figure 4). In a representative experiment, the unspiked baseline throughput was 454 L/m2 at 54% flow decay. The ultrapure preparation had no negative hydraulic impact at a spike level of 4 × 105 PFU/mL, with a throughput of 495 L/m2 at 50% flow decay. Moreover, a 10-fold higher spike to 5 × 106 PFU/mL was also harmless with respect to throughput, reaching 508 L/m2 at 52% flow decay. Contrast that with the ultracentrifuged preparation, which fouled the filter rapidly when spiked at only 2 × 105 PFU/mL, reducing throughput to 232 L/m2 at 95% flow decay.
Characterization predicted superior performance of the ultrapure virus stock. Total protein measurement and SDS-PAGE analysis revealed extremely low protein content. The scant protein impurities combined with the hi gh stock titer made membrane-fouling interactions with the feed material less likely, even at high spike concentrations. Testing confirmed that expectation. An ultrapure spike 100-fold higher than that feasible with ultracentrifuged MVM did not negatively affect flux or throughput. Had this been a clearance study for regulatory filing, the higher spike level enabled by the ultrapure preparation could have increased the LRV claim of the virus filter by two logs or more. The LRV would of course be limited if virus passes through the filter. Assuming complete virus retention by the filter and a large-volume assay, this spike would allow a LRV claim of >7, far beyond the initial target. Spike Right Our study supports two intuitive conclusions. First, thorough characterization of virus spike preparations can help minimize the challenges faced in clearance studies by enabling better prediction of performance. Second, intensive purification of virus stocks allows spiking to greater concentrations, and assuming effective virus reduction, consequent increased LRV claims. Furthermore, when the quality of virus stocks is assured and clearance studies are not confounded by unforeseen spiking effects, best spiking practices can be aggressively pursued. Scientific principles, regulatory guidelines, and economic efficiency are all in agreement regarding virus spiking practices: maintenance of a representative model system, maximization of LRV, and establishment of confidence in clearance study results. Regulatory guidance documents endorse the principle that scaled-down models used for virus clearance testing should “represent as closely as possible the production procedure” (12). Perturbation of the performance of any virus-clearance step by spike impurities is detrimental to that objective. In the case of virus filtration, spike impurity can manifest as a decrease in throughput that is not representative and may even cause an artificial elevation in observed LRV (4). Integrity of the scaled-down model as an accurate simulation of the manufacturing process depends on throughput and LRV being dictated by the feed material and filtration device, not virus spike impurities. Using highly purified virus spikes keeps a model representative of large-scale processes and prevents the need for oversized filter membranes to compensate for spiking artifacts. A further regulatory doctrine is that virus spikes should be “as high as possible to determine the capacity of the production step to inactivate/remove viruses adequately” (13). As shown by our experiments with ultrapure virus, very clean, high-titer virus stocks fulfill that recommendation and enable demonstration of higher LRVs. Greater LRV claims increase the value of virus filters from both virus clearance and economic view points. It could even reduce the need for implementation of other virus clearance steps. That will be critical as the industry moves toward more compact production processes involving fewer steps for drug purification. Each operation in compressed processes will need to claim higher LRV for total virus clearance values to be maintained, and virus stocks such as the ultrapure MVM support this goal. Regulatory guidelines also stress that replication of results is a critical component for confidence in virus safety: “An effective virus removal step should give reproducible reduction of virus load shown by at least two independent studies” (12). Insufficiently characterized, impure virus stocks can be a major source of study-to-study variability and are often the primary suspect when erratic results occur. Spiking effects can also mask what might be true variances in either the feed or virus filter. Conversely, when virus preparations are clean and well controlled, reproduction of results becomes much more likely. An accumulated historic database of consistent results could be used to support implementation of modular or generic virus clearance strategies, streamlining the studies required for future regulatory filings. Ultimately, the results of a virus filter clearance study depend on the interaction of three players: drug process feed material, virus filtration device, and virus spike. Performance of an unspiked feed across a filter best represents the manufacturing process. Once such performance is determined to meet required targets, responsibility for maintaining integrity of a scaled-down model falls upon the virus spike. In the very rare case of an actual virus contamination, all virus that reach the filter — typically one of the last unit operations in the production process — would be as clean as the drug product itself. Injection of virus stock impurities late into drug purification is certainly not part of a realistic scenario. Spike-induced fouling is merely the most obvious symptom of a filtration model that has been corrupted by introduction of nonrepresentative substances. Data presented here are evidence that it is possible to maintain the integrity of a scaled-down system with highly purified virus. Scientific validity of a clearance study is paramount, but the ability to achieve higher LRVs using less membrane area is certainly a welcome consequence as well. The industry has lived with virus spiking complications for quite some time, but technologies to improve virus stocks are available today. New methods can be used to raise the standards for virus clearance studies, and virus safety virus safety will advance when the industry takes that leap.
Author Details Corresponding author Damon R. Asher, PhD, is a senior scientist within the Bioprocess R&D Virology Group at EMD Millipore, 80 Ashby Road, Bedford, MA, 01730; 1-781-533-2554; damon.asher@merckgroup.com. Corresponding author Ashley L. Slocum is an applications engineer for the Biomanufacturing Sciences Network at EMD Millipore, 900 Middlesex Turnpike, Billerica, MA 01821; 1-781-533-5786; ashley.slocum@merckgroup.com. Katherine F. Bergmann, PhD, is the manager of Viral Safety and Viral Clearance Services at Lancaster Laboratories, Inc., Lancaster, PA. Paul Genest is a senior applications engineer for the EMD Millipore Biomanufacturing Sciences network. Amanda B. Katz is a scientist within the EMD Millipore Bioprocess R&D Virology Group. Jason J. Morais is a senior research associate, and Catherine M. Lawrence is a senior scientist in the Purification Process Research Group at Genzyme Corporation, Framingham, MA. Patricia Greenhalgh, PhD, is the Manager of the Bioprocess R&D Virology Group at EMD Millipore.
REFERENCES
1.) Bolton, G. 2005. Norma l-Flow Virus Filtration: Detection and Assessment of the Endpoint in Bioprocessing. Biotechnol. Appl. Biochem. 42:133-142.  2.) Cabatingan, M. 2005. Impact of Virus Stock Quality on Virus Filter Validation. BioProcess Int. 3:S39-S43.  3.) Denton, A, C Jones, and K. Tarrach. 2009. Integration of Large-Scale Chromatography with Nanofiltration for an Ovine Polyclonal Product. Pharm. Technol. 33:62-70.  4.) Khan, NZ. 2009. Filter Preconditioning Enables Representative Scaled-Down Modeling of Filter Capacity and Viral Clearance By Mitigating the Impact of Virus Spike Impurities. Biotechnol. Appl. Biochem. 52:293-301.  5.) Lute, S. 2007. Phage Passage After Extended Processing in Small-Virus-Retentive Filters. Biotechnol. Appl. Biochem. Erratumin: Biotechnol Appl. Biochem. 48(Pt 1) 2007: 63. 47:141-51.  6.) Wu, Y. 2009. Validation of Adventitious Virus Removal By Virus Filtration. BioProcess Int. 6:54-59.  7.) Garnick, RL. 1996. Experience with Viral Contamination in Cell Culture. Dev. Biol. Stand 88:49-56.  8.) Garnick, RL. 1998. Raw Materials As a Source of Contamination in Large-Scale Cell Culture. Dev. Biol. Stand. 93:21-29.  9.) 2000.Technical Report No. 47: Preparation of Virus Spikes Used for Virus Clearance Studies, Parenteral Drug Association.  10.) Astell, CR. 1983. The Complete DNA Sequence of Minute Virus of Mice: An Autonomous Parvovirus. Nucleic Acids Res. 11:999-1018.  11.) Willwand, K, and B. Hirt. 1993. The Major Capsid Protein V P2 of Minute Virus of Mice (MVM) Can Form Par ticles Which Bind to the 3′-Terminal Hair pin of MVM Replicative-Form DNA and Package Single-Stranded Viral Progeny DNA. J. Virol. 67:5660-5663.  12.) ICH 1998. Harmonized Tripartate Guideline: Q5A Viral Safety of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. Fed. Reg www.ich.org/LOB/media/MEDIA425.pdf. 63:51074.  13.) The European Agency for the Evaluation of Medicinal Products (EMEA) Human Medicines Evaluation Unit CPMP Biotechnology Working Party Note for Guidance on Virus Validation Studies: The Design, Contribution and Interpretation of Studies Validating the Inactivation and Removal of Viruses.