In the biopharmaceutical industry, continuous manufacturing is often cited as a method for increasing the productivity of bioprocesses (1). Compared with batch processing, it has the potential to enable production of more product within a smaller facility footprint — while improving product quality, particularly for sensitive and unstable molecules. Investigation into continuous methods is taking place for both upstream and downstream operations. For the full benefit of continuous processing to be realized, an argument has been made that cell culture,…
Upstream Single-Use Technologies
Performance Qualification of a Single-Use, Stirred-Tank Bioreactor with CHO-S Cell Culture
The increasing role and importance of cell culture in biophamaceutical manufacturing has led to considerable research and development (R&D) into bioreactor design and performance in recent years. As a result, a greater understanding of bioreactor fluid dynamics and critical physical parameters is now essential to maximize cell growth and productivity. Stirred-tank bioreactors are especially important in this development process because of their favorable properties in areas such as mixing efficiency and homogeneity, energy transfer, and scalability. The design and manufacture…
A Single-Use Process for Production of Recombinant Human Follicle-Stimulating Hormone
Follicle-stimulating hormone (FSH) is a heterodimeric glycoprotein consisting of noncovalently linked α and β subunits. It stimulates the growth of immature follicles in ovaries and primary spermatocytes in testes and thus plays an important role in human reproduction (1). Human menopausal gonadotropin for infertility treatment was first introduced into clinical practice in 1950 (2, 3). Subsequently, treatments with urinary FSH have been replaced by recombinant human FSH (rh-FSH), which has been shown to provide several advantages such as absence of…
Monitoring Live Biomass in Disposable Bioreactors
Often simply referred to as capacitance, radio-frequency (RF) impedance has been used for over two decades to measure online biomass. It is generally regarded as the most robust and reliable method to monitor live-cell concentrations in mammalian cell culture (1). Many biopharmaceutical companies have now made the transition from conventional glass or stainless steel multiuse (MU) vessels to single-use (SU) bioreactors. Disposables are rapidly becoming the preferred platform for new processes requiring current good manufacturing practice (CGMP) compliance. At the…
Evaluating New Film for Single-Use Bags: Growth Performance Studies with Animal and Human Cells
In biopharmaceutical development and manufacturing processes, single-use technology has become widely accepted (1). Storage and cultivation bags are particularly common. They are fabricated from plastics consisting of multilayer films and are typically provided gamma-sterilized by suppliers (2). The bags offer several advantages such as savings in time and cost. Lowered contamination risk results from reduced cleaning and sterilization demands. However, some adverse effects of polymer films on cell growth and metabolism have been reported, both for storage and cultivation bags…
The Case for a Standardized Assay to Test Suitability of Single-Use Systems in Cell Culture Applications
Increased commercial use of single-use systems (SUS) for large-scale biopharmaceutical production creates the need for consensus on industry best practices and standards for materials in SUS components. End users and suppliers are beginning to develop a shared vision of industry needs in such areas (1, 2). For example, highly visible efforts to harmonize extractables testing include contributions from groups such as the BioPhorum Operations Group (BPOG), Bio-Process Systems Alliance (BPSA), Parenteral Drug Association (PDA), ASTM, and ISPE. In addition to…
Single-Use Processing for Microbial Fermentations
During the past decade, single‑use bioprocessing has emerged as a standard platform for current good manufacturing practice (CGMP) mammalian cell culture. Biomanufacturers have come to appreciate the benefits of lower capital and operating costs, reduced contamination risk, continuity from early development through manufacturing, flexibility, and sustainability (1). Disposable cell‑culture vessels have gained wide acceptance because their performance duplicates that of stainless‑steel, fixed‑tank bioreactors, with which manufacturers have extensive experience. This is no accident: Single‑use bioreactors use stainless–steel engineering principles, particularly…
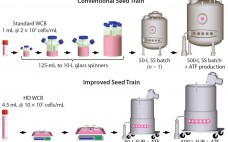
A Novel Seed-Train Process: Using High-Density Cell Banking, a Disposable Bioreactor, and Perfusion Technologies
A typical cell culture process begins with thawing of a cryopreserved cell-bank vial, followed by successive expansions into larger culture vessels such as shake flasks, spinners, Wave bags, and stirred bioreactors (1). When culture volume and cell density meet predetermined criteria, the culture is transferred to a production bioreactor in which cells continue to grow and express product. This approach presents several challenges. Shake flasks or spinners used in the initial stages require manual manipulations inside a laminar flow hood,…
Culturing a Duck ES-Derived Cell Line in Single-Use Bioreactors: A Rapid, Efficient, and Cost-Effective Vaccine Manufacturing System Based on Suspension Culture
Cell substrates managed in controlled culture environments have become, over the past few decades, the subject of intensive technological developments for the biomanufacturing of viral vaccines. The driving force of such work is an expanding demand for safety, high production capacities, cost savings, and flexibility. Egg, tissue, and primary-cell–based manufacturing methods of limited capacity are now considered to be outdated technologies. In the influenza vaccine field, for example, time delays in vaccine delivery (especially during pandemic responses) have increased concerns…
Measuring Pressure at Very Low Levels with High Accuracy in Single-Use Systems: Improved Performance and Single-Use System Testing
Measuring pressure in single-use systems (SUS) has become an integral part of both upstream and downstream bioprocess operations. Articles have been published on filtration applications (1), and integration into other SUS has been widely adopted. Additionally, information is available on low-pressure applications such as how to prevent overpressurization in single-use bioreactors (2). However, as users and applications both become more sophisticated, improved performance is sought for low-pressure applications (<1 psi) such as in single-use bioreactors. The reasons are two-fold: First,…