Suitable scale-down perfusion systems generally have been unavailable for process development (PD) activities. Some commercially available systems require daily media exchanges. No such system performs in a way that accurately represents large-scale perfusion, and none maintains sufficiently high cell densities. Kevin Lee (cofounder of Erbi Biosystems) joined BPI on 4 May 2021 to explain how his company’s Breez bioreactor system integrates all the functions of a stirred-tank reactor (STR) into a compact format that can facilitate PD, enabling one person…
Perfusion Cell Culture
Enabling High-Performing
Perfusion Cell Culture
At least one report estimates that by 2025, 35% of biologics will be manufactured using enhanced processes, particularly perfusion-based bioprocessing. Results of two innovations that support this need for enhanced processes are examined below. The Thermo Scientific HyPerforma DynaDrive Single-Use Bioreactor (SUB), the latest advancement in SUB technology, offers better performance than other bioreactors and scalability up to 5,000 L. Gibco High-Intensity Perfusion CHO Medium is formulated to provide exceptional performance and ease of use, with capability of sustaining >1…
Increasing Expression Titers: New Technologies Could Help Other Cell Lines Catch Up to CHO
Fang Tian is a lead scientist and head of cell biology research and development at the American Type Culture Collection (ATCC) in Manassas, VA. She is a member of both the International Cell Line Authentication Committee (ICLAC) and the US technical advisory group for the ISO/TC276 technical committee. At ATCC, she oversees preparation, authentication, characterization, quality control, and cryopreservation of more than 3,400 accessioned animal cell lines and hybridomas in the cell biology general collection. She holds a PhD in…
Product Quality Attribute Shifts in Perfusion Systems, Part 2: Elucidating Cellular Mechanisms
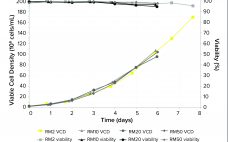
Part 1 of this two-part report describes an investigation into the potential cause(s) and ways to control a product quality attribute (PQA) of a protein expressed in perfusion cell culture (1). The presence of low–molecular-weight (LMW) species following size-exclusion high-performance liquid chromatography (SEC-HPLC) is a protein quality attribute that can indicate an increase in truncated forms of the expressed protein and/or other LMW moieties. The expressed protein in this study is a heavily glycosylated recombinant glycoprotein (rGP) comprising two subunits:…
Intensified Seed Train Strategy for Faster, Cost-Effective Scale-Up of Biologics Manufacturing
The high costs of and limits on global accessibility of biologics such as monoclonal antibodies (MAbs) are focusing the biopharmaceutical industry’s attention on strategies for rapid, economical development of such therapies. Process intensification is one approach to help shorten manufacturing timelines and reduce cost of goods (CoG) (1, 2). Today, process intensification in upstream cell culture enables biologics manufacturing in facilities with smaller footprints and lower scale-up volumes than was possible before. Intensified processing of Chinese hamster ovary (CHO) clones…
Product Quality Attribute Shifts in Perfusion Systems, Part 1: Identifying Shifts When They Occur
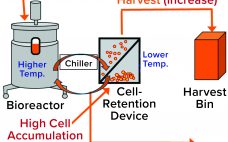
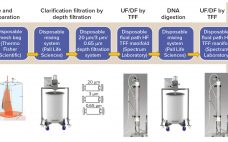
Perfusion cell culture processes are continuous, with fresh media continuously added and spent media (harvest) removed simultaneously through a cell-retention device (Figure 1). To maintain specific bioreactor cell density, cells are removed periodically as cell bleed or discard. Perfusion systems offer a number of advantages over batch and fed-batch culture modes such as lower capital costs and an ability to support higher cell densities with better viability over longer manufacturing campaigns requiring shorter turn-around times. However, perfusion systems require complex…
Continuous Biomanufacturing Implementation: Using an Intensified and Integrated Bioprocess Platform
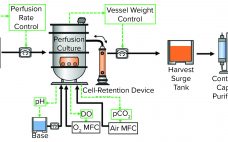
Recent world events have demonstrated now more than ever the growing demand for pharmaceutical biologics that can be made rapidly and in high volumes yet somehow remain affordable. Hence, there is an urgent need to develop a next-generation biomanufacturing solution that provides high-yield, high-quality drug products and is highly flexible and cost-effective. Herein we describe the WuXi Biologics ultrahigh productivity platform (WuXiUP), an intensified perfusion culture process developed to meet the aforementioned need. WuXiUP adopts process-intensification strategies on to traditional…
The Upstream Perspective: Taking Efficiency Beyond Cell-Line Development
With 20 years of experience in the biopharmaceutical industry — at Genentech, Applied Biosystems, Cell Genesys, Cellerant Therapeutics, and Bayer — Yuval Shimoni has written frequently for BioProcess International on a number of production topics. Those have ranged from process improvements and bioreactor scale-down validation, to raw materials management, to addressing variability and virus contamination events. For this featured report, we discussed hardware and instrumentation, quality by design (QbD) and related approaches, and other strategies that can take expediting upstream…
Advances and Challenges in Vaccine Development and Manufacture
Scientists have made significant breakthroughs in bioprocess and analytical technologies for supporting vaccine development. Such technologies have helped vaccine manufacturers achieve consistent product purity and quality rapidly and cost effectively. Although interest in vaccine development and manufacture continues to increase because of the rapid growth of the global vaccine market, this area of the bioprocess industry remains challenging and complex. Here we review the current constraints and complexities in the vaccine industry, specifically related to product development and manufacture. We…
Cell Culture Bioprocessing in Perfusion: Assessing Cell Retention Technologies
Upstream bioprocessing in perfusion mode holds great promise for industrial production of cells and biologics. In perfusion, fresh medium is added constantly to the bioreactor, and used medium is harvested while the cells are retained in the bioreactor. As a result, the composition of the cell culture medium stays quite constant during the process. This offers several advantages. In perfusion, higher cell densities can be reached than in batch and fed-batch processes, therefore enhancing volumetric productivity. Because medium composition can…