Adenoassociated virus (AAV) vectors are a popular choice for modern gene therapies because of their favorable safety profile, low immunogenicity, and the ease with which they can be transduced into different cell and tissue types. An AAV genome is a single strand of DNA comprising a replication (rep) gene, which encodes regulatory proteins involved in genome replication, and a capsid (cap) gene, which produces three capsid proteins. However, AAVs cannot replicate alone. In nature, AAV shares an exquisite relationship with…
Upstream Processing
eBook: Raw Material Control Strategy — Leveraging Knowledge of Material Attributes and Data Analytics as Key Elements
Ensuring pharmaceutical quality begins with in-depth understanding of process/platform capabilities, which is informed by knowledge gained through product and process development, subject-matter expertise, and lessons learned from experience. And all outside factors that can affect manufacturing outcomes must be taken into consideration. Extra vigilance is necessary for understanding potential sources of variation and maintaining robust control strategies to ensure process consistency — and ultimately product quality for patients. Biomanufacturing unit operations require multiple raw materials that must be documented as…
Updating the Economics of Biologics Manufacturing with 5,000-L Single-Use Bioreactors: A Paradigm Shift
Single-use technologies enable a flexibility and modularity effectively unattainable with more traditional stainless-steel technologies, particularly in upstream bioprocesses. Single-use bioreactors up to 2,000 L are employed largely in preclinical- and clinical-stage bioprocesses to leverage this flexibility. As products reach commercial maturity, scales larger than 2,000 L frequently become desirable to take advantage of economies of scale. With the typical upper limit of single-use bioreactors at 2,000 L, this has traditionally meant transfer to stainless-steel systems. The introduction of the Thermo…
Using Peptones to Achieve Diverse and Demanding Bioproduction Goals
As bioproduction requirements advance, it is critical to have consistent, high-quality media and supplements that continue to meet evolving industry needs. Peptones have been successfully used in bioproduction applications for more than 30 years to meet diverse and demanding production requirements. Their unique nutritional profiles and usage flexibility make peptones ideal components for creating a robust bioprocess. This Special Report will demonstrate the benefits of peptones and how they can be used to enhance process performance and consistently yield a…
Optimizing Cell Line Development for High-Quality Biologics
For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. Online presentations from the CLD track of the BioProcess…
Plant-Cell Cultures and Cell Lines for Recombinant Protein Expression
Cell cultures derived from mammalian and bacterial cell lines are the conventional production systems in bioprocessing. But they also have their limitations. Media for mammalian cultures in particular are notoriously expensive, and traditional cell cultures can be highly sensitive to growing conditions. During the late 1980s and into the 1990s, plants and plant-derived cell cultures were introduced as alternative cell-culture systems (1, 2). Although transgenic plants (genetically modified) once looked promising in the early 2000s, the cost and manufacturing complexity…
Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization
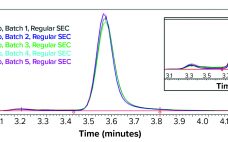
Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin G (IgG) product integrity in real time during a bioreactor run, especially…
Toward a Roadmap for Cell-Free Synthesis in Bioprocessing
Cell-free synthesis (CFS), also known as cell-free transcription and translation, supplements cellular components (either a cell lysate or purified recombinant elements) with nucleotides, amino acids, metabolic intermediates, and salts to produce a nucleic acid or protein from a genetic template added to the reaction. This exciting technology has seen a substantial increase in both academic and commercial interest over the past decade (1). Interest stems in large part from the potential to democratize access to the machinery of biology by…
Product Quality Attribute Shifts in Perfusion Systems, Part 1: Identifying Shifts When They Occur
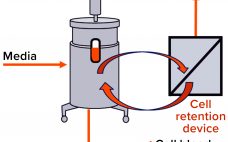
Perfusion cell culture processes are continuous, with fresh media continuously added and spent media (harvest) removed simultaneously through a cell-retention device (Figure 1). To maintain specific bioreactor cell density, cells are removed periodically as cell bleed or discard. Perfusion systems offer a number of advantages over batch and fed-batch culture modes such as lower capital costs and an ability to support higher cell densities with better viability over longer manufacturing campaigns requiring shorter turn-around times. However, perfusion systems require complex…
Microbial Expression and Purification: One Company’s Historical Perspective
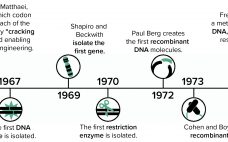
Since the dawn of the recombinant DNA era in the 1970s, New England Biolabs (NEB) has been integrally involved in expressing and purifying proteins, both for its own research interests and for biomanufacturing processes. In 1978, the company began screening microorganisms for restriction enzymes. Our scientists remember the challenges met in purifying limited amounts of restriction enzymes and other proteins from native organisms isolated from the environment. The efforts of those scientists to clone, overexpress, and purify restriction enzymes from…