Process development teams have relied for decades on benchtop bioreactors to perform mammalian-cell–culture experiments; however, doing so requires significant resources and staffing. During a 24 February 2021 presentation, Deborah Pascoe, PhD, vice president of operations at Culture Biosciences (Culture), explained how to leverage her company’s high-throughput, cloud-connected bioreactors to execute Chinese hamster ovary (CHO) cell cultures with high reproducibility and scalability. Pascoe’s Presentation Pascoe described her company as an extension of a customer’s laboratory. After technology transfer, Culture and a…
Upstream Processing
Introduction: Technologies Converge in Biopharmaceutical Laboratories
Bioassay development is foundational to the well-characterized biotechnology product paradigm. Bioassays are the best tools for drug developers to use in determining the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Thus, these assays are vital to quality assurance and quality control (QA/QC), preclinical studies, and clinical testing — and by extension to process development and monitoring. Because of their complex nature, bioassays are among…
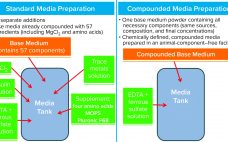
Compounded Media Powder Streamlines Cell Culture Media Preparation Operations
Cell culture medium is critical to cell growth, metabolism, and protein expression. It provides for optimum pH, osmolality, and nutrients in an environment that is essential for cell survival, growth, and expression of proteins and/or metabolites and drug-substance modalities of interest (1). A complete medium typically contains basic nutrients such as carbohydrates, amino acids, lipids, salts, vitamins, trace metals, growth factors/hormones (e.g., insulin), antishear factors, and other chemicals that facilitate cell growth and protein expression and may stabilize recombinant protein…
Fetal Bovine Serum Source Countries: Comparing Regulatory Animal Health Infrastructures
The motto of the European Serum Products Association (ESPA) — “Serum Saves Lives” — reaffirms the essential role that animal serum plays in cell-culture–based research and applications to protect the health of both human and animal populations. Animal serum, especially fetal bovine serum (FBS), needs to be available in abundant supply and at affordable prices to medical and veterinary facilities all over the world. With one billion cattle globally, the supply of FBS should be plentiful, but not all countries…
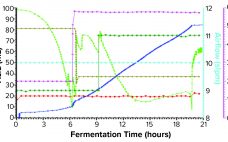
Oxygen Control Strategy and Yield of Recombinant Antibody Fragments Produced in Fermentation
Immunoglobulin molecules are used extensively in therapeutic treatments, diagnostic applications, and fundamental academic research. Traditionally, full-length antibodies and smaller fragments such as the recombinant antigen-binding fragment (rFab) are produced through mammalian cell culture. rFabs also are small enough to be produced in Escherichia coli through fermentation (1, 2). Because disulfide bonds cannot be formed efficiently in the reducing cytoplasm of E. coli, rFabs are supplemented most commonly with a signal sequence that directs them to the more oxidizing bacterial periplasm…
Bioreactor Automation Driven by Real-Time Sensing: Enhancing Productivity Through Accurate, Efficient Glucose Control
In the quest for improved quality and productivity in drug manufacturing, the industry is moving toward increasing use of bioreactor systems with real-time integrated monitoring and advanced analytics that can enable automation, drive performance, and improve data-rich quality control. However, there are multiple options for sensors and technologies that monitor important cell-culture variables or critical process parameters (CPPs). Furthermore, cell culture vessels can be disposable single-use bioreactors (SUB) or reusable glass or stainless-steel models. They can operate in stirred tanks,…
Reducing Risk in Bioproduction with Facilities Equivalency
Providing consistency in cell culture media biomanufacturing is critical to supply continuity. Central to this is the development of redundancy and harmonization across a global manufacturing network. These unprecedented times have also highlighted the importance of strategizing for increased and unexpected demand. Read this Special Report to learn about the importance of equivalency and the strategies used to maintain this critical requirement at Gibco cell culture media manufacturing facilities. Fill out the form below to read the complete report and…
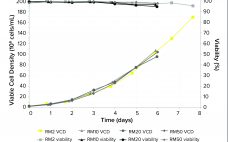
Product Quality Attribute Shifts in Perfusion Systems, Part 2: Elucidating Cellular Mechanisms
Part 1 of this two-part report describes an investigation into the potential cause(s) and ways to control a product quality attribute (PQA) of a protein expressed in perfusion cell culture (1). The presence of low–molecular-weight (LMW) species following size-exclusion high-performance liquid chromatography (SEC-HPLC) is a protein quality attribute that can indicate an increase in truncated forms of the expressed protein and/or other LMW moieties. The expressed protein in this study is a heavily glycosylated recombinant glycoprotein (rGP) comprising two subunits:…
Intensified Seed Train Strategy for Faster, Cost-Effective Scale-Up of Biologics Manufacturing
The high costs of and limits on global accessibility of biologics such as monoclonal antibodies (MAbs) are focusing the biopharmaceutical industry’s attention on strategies for rapid, economical development of such therapies. Process intensification is one approach to help shorten manufacturing timelines and reduce cost of goods (CoG) (1, 2). Today, process intensification in upstream cell culture enables biologics manufacturing in facilities with smaller footprints and lower scale-up volumes than was possible before. Intensified processing of Chinese hamster ovary (CHO) clones…
Cryopreserved Leukapheresis Materials Help Alleviate Donor Sourcing Issues
Reliable access to high-quality starting material is a primary challenge in cell therapy manufacturing. Freshly isolated leukopak starting materials depend on donor availability and are vulnerable to cell losses from scheduling changes and other unforeseen circumstances. Flexibility often is required for cell therapy manufacturing. Therefore, relying solely on freshly isolated starting material is impractical, particularly when cell therapy logistics involve global shipping and distribution. Donor sourcing is among the most critical factors shaping cell and gene therapy (CGT) supply chains.…