Through the past decade, single-use bioreactors for culturing mammalian and insect cells have been widely adopted in preclinical, clinical, and production-scale biopharmaceutical facilities (1, 2). With such bioreactors in operation, monitoring and control of process parameters is vital for ensuring critical quality attributes (CQAs) of biologicals or vaccines are met for production of a safe product. Traditionally, bag-based and bench-top vessels have been fitted with conventional pH and dissolved oxygen (DO) probes similar to those used in stainless steel or…
Upstream Processing
Consistently Superior Cell Growth: Achieved with New Polyethylene Film Formulation
During the past decade, single-use bioprocessing bags and bioreactors have gained a significant foothold in the biopharmaceutical industry because they offer a number of advantages over traditional stainless steel equipment, especially for clinical production, multiproduct facilities, and emerging economies. At the same time, some companies are concerned that plastic materials might release potentially toxic substances that could affect cell growth and product titers (1). In a worst-case scenario, they could even compromise drug safety when a company uses disposable bags…
Development and Qualification of a Scalable, Disposable Bioreactor for GMP-Compliant Cell Culture
During the development of single-use, stirred-tank bioreactors (e.g., BIOSTAT STR bioreactors), different phases can be distinguished (Figure 1). First, a clear definition of the intended application and all related requirements should be captured in a user requirement specification (URS). Based on that, the single-use bioreactor design phase and the material selection phase are initiated, both closely linked to each other. During the proof-of-concept phase, relevant component- and product-based tests are established and realized to ensure URS compliance. Finally, the qualification…
Verification of New Flexsafe STR Single-Use Bioreactor Bags: Using a CHO Fed-Batch Monoclonal Antibody Production Process at 1,000-L Scale
In the past decade, single-use bioreactors have gained wide acceptance for biomanufacturing. The biopharmaceutical industry is increasingly interested in performing modern production processes in single-use facilities. That trend is driven by the time and cost benefits of single-use technologies, as well as the enhanced manufacturing flexibility they offer (1). With single-use bioreactors increasingly used in late-phase clinical trials and commercial production, their quality, reliability, and assurance of supply becomes more critical. Many industry experts consider process control of film and…
Pressure Decay Method for Postinstallation Single-Use Bioreactor Bag Testing
Single-use technology is well accepted today, and manufacturers’ quality assurance programs ensure leak-free single-use bags upon delivery. But what about risks involved with installation and other handling errors? Operator training and implementation of suitable standard operating procedures (SOPs) are mandatory, but should they be the only ways to mitigate the risk of failures? In addition, more companies are advocating the use of ballroom concepts (1) for the manufacture of biopharmaceutical drug substances and drug products. However, how do you prove…
Supply Chain Challenges in the Biopharmaceutical Industry: A Case Study Following the 2011 Tsunami in Japan
Global manufacturing of biopharmaceuticals for human use helps save the lives of millions of people and is a large commitment to public health. The industry operates in an environment with financial uncertainties and complex international supply chains, so the question of risk mitigation is paramount. There is an expectation that comprehensive risk mitigation programs should be in place to minimize the risk of supply chain interruptions that would negatively affect the manufacture of these vital therapeutics. Here we share how…
Fed-Batch Cell Culture Process Development: Implementing a Novel Nutrient Additive for a Robust, High-Titer, Scalable Process
The fed-batch culture of Chinese hamster ovary (CHO) cells has become well established as the primary method of manufacturing therapeutic recombinant protein products for various disease indications. Fed-batch process-development approaches focus on supporting high–cell-density cultures that are crucial to achieving high product titers but lead to proportionately high nutritional demands. Exhaustion of key nutrients negatively affects cell growth and ability to produce recombinant proteins. To counter that problem, concentrated feeds are added to the culture. Such feeds tend to be…
A Novel Solid-Media E. coli Platform: Comparison with Standard Fermentation Processes
MicroProtein Technologies Inc. has developed the MPTxpress high-yield, low‑cost, recombinant Escherichia coli manufacturing platform. Rather than using liquid culture media within stirred bioreactors, the system uses trays filled with semisolid (gelled) culture media overlaid with or without a permeable membrane on which the E. coli is cultured. Compared with conventional liquid fermentation platforms, the MPTxpress system reduces the number of steps in up- and downstream processing and required infrastructure, significantly improves yields, and lowers costs. It provides simplicity for mixing…
Industry Experts Convene in New York to Discuss Latest Innovations: A BPI Special Report
As the biopharmaceutical industry continues to mature and grow, so too does the need to educate a broader audience of biopharmaceutical professionals interested in hearing, understanding, and applying the latest science and technology trends that support and in many cases are transforming today’s bioprocesses. To reach this extended and engaged audience, BioProcess International created the BPI Theater Series: a live, interactive program that provides bioprocessing content to traditional, noncore biopharmaceutical conference programs. It provides attendees with the opportunity to interact…
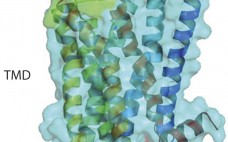
Targeting G Protein–Coupled Receptors with Biologics for Therapeutic Use, Part 1
G -protein coupled receptors (GPCRs) represent a target superfamily linked to many disorders across all therapeutic areas. Although this target class has been historically treated by small molecules and peptides, antibodies can offer a number of advantages over such molecules by virtue of their specificity, dosing frequency, and restricted penetration. They also can provide other functional effects specifically mediated by the Fc region (ADCC and CDC) as well as different modalities such as those offered by bispecific and antibody drug…