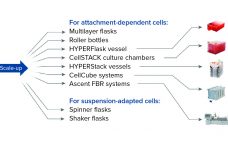
When considering strategies for expanding the number of cells being grown to support cell therapy development, companies often focus on decisions regarding scale-up and scale-out: increasing capacity either by using larger vessels to increase production volume or by implementing more units of the same vessel, respectively. Complete workflows often involve both. Figure 1 shows an example of scaling out from one to multiple cell culture flasks of the same dimension before transitioning to a larger format. Scale-out can be straightforward…
Upstream Processing
Ask the Expert: Facilitating Process Development Using a Microfluidic Perfusion Bioreactor
Suitable scale-down perfusion systems generally have been unavailable for process development (PD) activities. Some commercially available systems require daily media exchanges. No such system performs in a way that accurately represents large-scale perfusion, and none maintains sufficiently high cell densities. Kevin Lee (cofounder of Erbi Biosystems) joined BPI on 4 May 2021 to explain how his company’s Breez bioreactor system integrates all the functions of a stirred-tank reactor (STR) into a compact format that can facilitate PD, enabling one person…
Ask the Expert: A Novel Escherichia coli System for Controlled Release of Difficult-to-Secrete Proteins
Although Escherichia coli often enables dependable, highly productive expression of nonglycosylated recombinant proteins, the efficiency with which it secretes a target protein into culture supernatant can depend greatly on that molecule’s physicochemical properties. Some proteins remain trapped in periplasm, thus diminishing process yield and productivity. In June 2021, Marcel Thoen (head of Wacker Biotech’s Global Competence Center for Cell Line Development) described how his company’s improved ESETEC (E. coli secretion technology) solutions can address productivity challenges raised by difficult-to-secrete recombinant…
Ask the Expert: Leveraging Quality Management Systems to Achieve Competitive Advantages
During a May 2021 “Ask the Expert” presentation, Jigisha Patel (vice president of global regulatory compliance and technical services at Spectrum Chemical Manufacturing Corporation) emphasized the need to minimize supply-chain contingencies that lead to variability across raw materials used in biopharmaceutical manufacturing. Deviations in raw-material specifications can jeopardize good manufacturing practice (GMP) compliance and reduce drug-product efficacy and stability. Patel explained how her company’s quality management system ensures that bioprocess materials will meet specifications and process needs. Patel’s Presentation Patel…
High-Yield AAV Viral Vector Production in Corning Ascent Fixed-Bed Reactor System
Gene therapies hold great promise for one-time treatments that alleviate or cure certain genetic conditions. Recombinant adenoassociated viral (AAV) and lentiviral vectors have emerged as leading gene delivery methods for in vivo and ex vivo gene therapies, respectively. With more than 500 gene therapy clinical trials underway, the industry needs scalable, cost-efficient viral vector manufacturing. To address those needs, Corning developed the Ascent fixed-bed bioreactor system for scalable, high-density, adherent cell culture, including high-yield viral vector manufacturing. The Ascent fixed-bed…
Model-Based Process Monitoring of Algae Cultures Using eve Software
In bioreactor processes, the on-line monitoring of key parameters relies on the use of adequate sensors. In this article, we implement model-based software sensors to monitor nitrate concentrations in an algae batch process. Nitrate concentration is a crucial component in algae growth, and its assessment is used to achieve optimal growth conditions or to determine optimal harvest times. Batch cultivation of the algae Chlamydomonas reinhardtii is carried out in a Labfors 5 airlift photobioreactor provided by INFORS-HT. By means of…
Enabling High-Performing
Perfusion Cell Culture
At least one report estimates that by 2025, 35% of biologics will be manufactured using enhanced processes, particularly perfusion-based bioprocessing. Results of two innovations that support this need for enhanced processes are examined below. The Thermo Scientific HyPerforma DynaDrive Single-Use Bioreactor (SUB), the latest advancement in SUB technology, offers better performance than other bioreactors and scalability up to 5,000 L. Gibco High-Intensity Perfusion CHO Medium is formulated to provide exceptional performance and ease of use, with capability of sustaining >1…
Take Complexity and Risk Out of Regulatory Compliance
Novo Nordisk Pharmatech’s high-purity, nontherapeutic insulin is sourced directly from the Novo Nordisk parent company, the world’s largest insulin producer. The product consists of insulin human crystals that are biosynthetically produced by recombinant microbial expression in yeast. Insulin human AF stimulates the proliferation of cells and enhances the yield, and it is a key component in serum-free growth media for mammalian cells. Insulin human AF is used for manufacturing monoclonal antibodies, virus vaccines, gene therapies, and other biological drug products…
Contractor Perspectives: Best Practices for Transfer, Handling, Testing, and Storage of Cell Banks
For comments about how contract development and manufacturing organizations (CDMOs) manage their cell-banking quality assurance (QA) practices. I contacted long-time member of BPI’s Editorial Advisory Board Scott M. Wheelwright, PhD, for his perspectives. Wheelwright brings many years of experience to this discussion, with insights into the evolution of technologies and practices extending back to the early launch of the biopharmaceutical industry. Currently, he provides consulting support for companies with manufacturing and sourcing in China and other Asian countries. He also…
Raw Materials for Advanced Therapies: When the Process Is the Product, Ingredients Are Key
Scott Burger and Bill Janssen are both established, independent consultants specializing in gene and cell therapies. This past spring, we three discussed several aspects of raw material strategy for advanced therapies as well as the need for trained technicians in the industry. With a bachelor of science degree in biology from Tulane University (New Orleans, LA, 1983) and a medical doctorate from the University of Pennsylvania (Philadelphia, PA, 1988), Scott Burger served his residency training and fellowship at Washington University…