The benefits of single-use technologies in both upstream and downstream operations are now widely acknowledged by the biopharmaceutical industry, and have led to radical changes in the design and operation of many bioprocesses. Those changes typically provide more robust processes and increased production flexibility. For mammalian cell culture, cleanable multiuse glass or stainless steel stirred-tank reactors (STRs) have been used successfully for growth of suspension-adapted cell lines in both small- and large-scale systems. However, achieving the same or better performance…
Upstream Processing
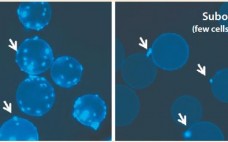
Strategies for Microcarrier Culture Optimization
The process of delivering an allogeneic stem-cell therapy to patients requires isolation and expansion of rare tissue-specific stem cells, which are subsequently delivered to individual patients for treatment. One type of cell used for such therapies is commonly known as human mesenchymal stem cells (hMSCs). They have been isolated from a number of tissues: e.g., bone marrow, heart, brain, placenta, and umbilical cord. And they have been shown to be immune-privileged in that hMSCs elicit no graft-versus-host (GvH) response such…
Assessing Similarity with Parallel-Line and Parallel-Curve Models: Implementing the USP Development/Validation Approach to a Relative Potency Assay
Potency is a critical quality attribute to support development and release of biopharmaceutical products. Researchers assess most protein-drug potencies using biological assays (such as cell-based assays), which mimic a product’s known mechanism of action or binding assays (if the only known mechanism of action is a drug binding to its target or if a drug is in early phases development). Potency denotes an important feature of complex biologics: their biological activity produced as a direct result of the molecule’s tertiary/quaternary…
Bioreactor Design for Adherent Cell Culture — The Bolt-On Bioreactor Project, Part 3: Containment, Sterility
The Bolt-on Bioreactor (BoB) project is an independent initiative aimed at developing and commercializing a bioreactor for the automated and efficient culture of adherent cells, especially for application in the production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must answer four major challenges. Addressed in the first article of this series (2), the first challenge has to do…
Bioreactor Design for Adherent Cell Culture: The Bolt-On Bioreactor Project, Part 2 — Process Automation
The Bolt-on Bioreactor (BoB) project is an independent initiative to develop and commercialize a bioreactor for automated and efficient culture of adherent cells, especially in production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges. Addressed in the first installment of this series (2), the first challenge concerns volumetric productivity. The second challenge is…
Single-Use Processing for Microbial Fermentations
During the past decade, single‑use bioprocessing has emerged as a standard platform for current good manufacturing practice (CGMP) mammalian cell culture. Biomanufacturers have come to appreciate the benefits of lower capital and operating costs, reduced contamination risk, continuity from early development through manufacturing, flexibility, and sustainability (1). Disposable cell‑culture vessels have gained wide acceptance because their performance duplicates that of stainless‑steel, fixed‑tank bioreactors, with which manufacturers have extensive experience. This is no accident: Single‑use bioreactors use stainless–steel engineering principles, particularly…
Culture Media and Protein Expression From Conversations with William G. Whitford
As part of BPI’s “Ask the Expert” series, editorial advisor William G. Whitford (senior technical market manager for GE Healthcare Life Sciences) spoke with editor-in-chief Anne Montgomery and marketing and digital content strategist Leah Rosin on two separate occasions about issues related to culture media and expression titers. Sourcing Serum-Free Media Anne discussed cell culture media and process fluids with Bill in March 2014. Whitford: Things are advancing, and the industry is changing significantly. In general, we are using more…
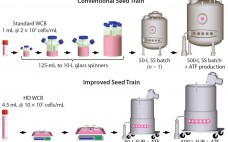
A Novel Seed-Train Process: Using High-Density Cell Banking, a Disposable Bioreactor, and Perfusion Technologies
A typical cell culture process begins with thawing of a cryopreserved cell-bank vial, followed by successive expansions into larger culture vessels such as shake flasks, spinners, Wave bags, and stirred bioreactors (1). When culture volume and cell density meet predetermined criteria, the culture is transferred to a production bioreactor in which cells continue to grow and express product. This approach presents several challenges. Shake flasks or spinners used in the initial stages require manual manipulations inside a laminar flow hood,…
Culturing a Duck ES-Derived Cell Line in Single-Use Bioreactors: A Rapid, Efficient, and Cost-Effective Vaccine Manufacturing System Based on Suspension Culture
Cell substrates managed in controlled culture environments have become, over the past few decades, the subject of intensive technological developments for the biomanufacturing of viral vaccines. The driving force of such work is an expanding demand for safety, high production capacities, cost savings, and flexibility. Egg, tissue, and primary-cell–based manufacturing methods of limited capacity are now considered to be outdated technologies. In the influenza vaccine field, for example, time delays in vaccine delivery (especially during pandemic responses) have increased concerns…
Measuring Pressure at Very Low Levels with High Accuracy in Single-Use Systems: Improved Performance and Single-Use System Testing
Measuring pressure in single-use systems (SUS) has become an integral part of both upstream and downstream bioprocess operations. Articles have been published on filtration applications (1), and integration into other SUS has been widely adopted. Additionally, information is available on low-pressure applications such as how to prevent overpressurization in single-use bioreactors (2). However, as users and applications both become more sophisticated, improved performance is sought for low-pressure applications (<1 psi) such as in single-use bioreactors. The reasons are two-fold: First,…