Raw material storage, handling, and processing are essential to ensure high product quality and consistent process performance. Slight variabilities in raw materials (either inherent in the material or through processing) can compromise yield and even result in batch loss. On Tuesday 26 September 2017 speakers at the BioProcess International Conference (part of Biotech Week Boston) addressed raw material variability and control strategies in biomanufacturing. They discussed the industry’s initiative for raw material risk assessments and strategies to control variability by…
Upstream Processing
eBook: SUStainability — Concerning Single-Use Systems and the Environment
Disposable materials have been used in many aspects of biomanufacturing since muromonab was first launched in 1986. Single-use stirred-tank bioreactors first became commercially available from HyClone in 2004 (1). Despite their demonstrated value to bioprocessing, disposable materials remain the subject of wide-ranging differences of opinion. Discussions of any technology are healthy and important for identifying areas for improvement, but some hearsay and bold propositions made regarding single-use components and the environment are not always helpful. Sustainability is an important and…
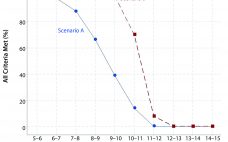
Statistical Assessments of Bioassay Validation Acceptance Criteria
Analytical linearity as well as assessments of precision and accuracy determine the range for a bioassay (1). USP <1033> recommends comparing confidence intervals (CIs) against target validation acceptance criteria in a bioassay validation exercise, but there are no clear guidelines for determining the criteria (2). Here I address several aspects of a bioassay validation, namely • Linearity (coefficient of determination (R2), slope, and intercept parameters) • Accuracy (%relative bias, %RB) • Precision (percent coefficient of variation, %CV) CIs for the…
eBook: The Commercial Expression Systems Market — What Has Changed in the Past Decade
A decade ago, BioPlan Associates prepared the findings of its 2008 directory of expression system technologies that were being promoted or considered likely to be suitable for commercial licensing for biopharmaceutical manufacturing (1). Due in part to the relatively slow advances in this critical area of bioprocessing, this study remains perhaps the only directory of biopharmaceutical-relevant expression systems available for licensing. Here I discuss aspects of related bioprocessing technologies that have and have not changed in the past decade. Expression…
eBook: Production Cell-Line Development and Control of Product Consistency During Cultivation — Myths, Risks, and Best Practices
Health authorities are requesting substantial details from sponsors regarding practices used to generate production cell lines for recombinant DNA–(rDNA) derived biopharmaceuticals. Authorities also are asking for information about the clonality of master cell banks (MCBs) and control strategies to minimize genetic heterogeneity. Such requests are prompted by recent reports indicating “nonclonality” for certain production cell lines. To address these and related issues, the CASSS CMC Strategy Forum on “Production Cell Line Development and Control of Product Consistency During Cell Cultivation:…
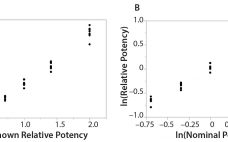
The Relationship Between R2 and Precision in Bioassay Validation
Analytical linearity along with assessments of precision and accuracy determine the range for bioassays (1). Practitioners can include coefficient of determination (R2) criteria from a linearity study in the bioassay validation protocol. Herein I illustrate the relationship of R2 to study design and analytical method variation. Overview of the Simple Linear Regression Model Dilutional linearity assesses the “ability (within a given range) of a bioassay to obtain measured relative potencies that are directly proportional to the true relative potency of the…
Cell Expansion with Dissolvable Microcarriers
In recent years we have seen an exponential increase in the number of companies testing and validating new regenerative medicine products. Many of these products are reaching late-phase trials with the potential to receive final approval and marketing authorization from regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). In the past several years, we have seen successful launches of regenerative medicine products, including Holoclar (Holostem Advanced Therapies), Kymriah (tisagenlecleucel, Novartis), Yescarta…
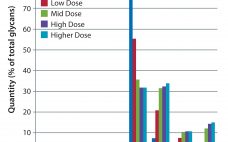
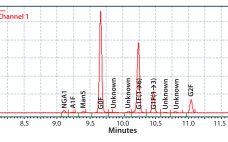
Enhanced Galactosylation of Monoclonal Antibodies: Using Medium Supplements and Precursors of UDP-Galactose, Part 2
In Part 1 of this report, we described our development of a high-throughput assay for analyzing monoclonal antibody (MAb) glycans and how we used it to evaluate the effects of medium supplements on galactosylation of MAbs produced by two different cell lines (1). This month, we examine galactosylation of a MAb produced by a third cell line. A discussion follows on the benefits of this high-throughput assay before we highlight the similarities and differences in galactosylation among the three MAbs…
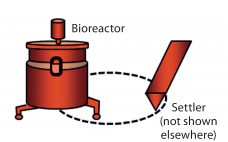
Reducing Variability in Cell-Specific Productivity in Perfusion Culture: A Case Study
Variation in bioproduction is recognized in the industry and often attributed to one or more of four sources: raw materials (including consumables), operational inputs (measurements, methods, personnel, equipment), environmental factors, and biological variation inherent to living cells (1). Variability can occur even among replicate units regardless of production mode (e.g., fed-batch or perfusion), and it can manifest as variability in productivity, cell metabolism, and/or product quality (2–4). In commercial biomanufacturing, meeting all product quality attributes is a requirement for regulatory…
Enhanced Galactosylation of Monoclonal Antibodies: Using Medium Supplements and Precursors of UDP-Galactose, Part 1
The biopharmaceutical industry needs better understanding of how monoclonal antibody (MAb) glycosylation is influenced by components in cultivation media — and it needs methods to exert some control over the structure of MAb glycans. That structure can affect MAb function. Thus, a high-throughput (HTP) assay is needed for characterizing MAb glycosylation so that developers can observe the effects of cultivation conditions on MAb glycosylation rapidly, with a goal of producing MAbs that have a desired glycan structure. The method also…