Cell cultures derived from mammalian and bacterial cell lines are the conventional production systems in bioprocessing. But they also have their limitations. Media for mammalian cultures in particular are notoriously expensive, and traditional cell cultures can be highly sensitive to growing conditions. During the late 1980s and into the 1990s, plants and plant-derived cell cultures were introduced as alternative cell-culture systems (1, 2). Although transgenic plants (genetically modified) once looked promising in the early 2000s, the cost and manufacturing complexity…
Expression Platforms
Toward a Roadmap for Cell-Free Synthesis in Bioprocessing
Cell-free synthesis (CFS), also known as cell-free transcription and translation, supplements cellular components (either a cell lysate or purified recombinant elements) with nucleotides, amino acids, metabolic intermediates, and salts to produce a nucleic acid or protein from a genetic template added to the reaction. This exciting technology has seen a substantial increase in both academic and commercial interest over the past decade (1). Interest stems in large part from the potential to democratize access to the machinery of biology by…
Microbial Expression and Purification: One Company’s Historical Perspective
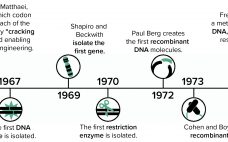
Since the dawn of the recombinant DNA era in the 1970s, New England Biolabs (NEB) has been integrally involved in expressing and purifying proteins, both for its own research interests and for biomanufacturing processes. In 1978, the company began screening microorganisms for restriction enzymes. Our scientists remember the challenges met in purifying limited amounts of restriction enzymes and other proteins from native organisms isolated from the environment. The efforts of those scientists to clone, overexpress, and purify restriction enzymes from…
eBook: Expression Systems — Innovative Techniques for Conventional Cell Lines
Although Chinese hamster ovary (CHO) and Escherichia coli cells have become the biopharmaceutical industry’s preferred platforms for producing recombinant proteins, perennial challenges have limited the capabilities of those expression systems. New CHO lines and improved upstream methods steadily are increasing expression titers, yet researchers continue to decry CHO’s relatively low growth rate. E. coli exhibits strong growth kinetics but cannot perform posttranslational modifications necessary for complex therapeutic proteins. Researchers need advanced technologies and analytical methods to overcome such limitations. This…
Application of Targeted Locus Amplification for Enhanced Apollo X CHO Clone Screening
Speed to market is an important consideration for the development of lifesaving therapies, including recombinant monoclonal antibodies. However, increased speed must be balanced with quality to enable quick and efficient delivery of biopharmaceuticals to patients. Important determinants of quality include the genomic location and integrity of the transgene sequence within a recombinant cell line. Traditional methods of genetic characterization can provide incomplete information and results can be difficult to interpret. However, next-generation sequencing (NGS) approaches such as Targeted Locus Amplification…
Get to IND Faster: Accelerated and High-Performance Cell-Line Development
In April 2020, Samsung Biologics hosted a webinar with John Gill, the company’s director of cell-line development (CLD). He focused first on chemistry, manufacturing, and controls (CMC) activities needed for preparing an investigational new drug (IND) application. Then he introduced a fast timeline for managing CLD programs to accelerate client projects to success. CMC Activities Samsung Biologics is a fully integrated contract development and manufacturing organization (CDMO). Starting with a client’s gene of interest (GoI), Gill’s group tailors CLD to…
Anticipating Cell-Line Challenges to Drive CMC Readiness
Development of a safe and high-quality Chinese hamster ovary (CHO) cell line is of paramount importance for the chemistry, manufacturing, and controls (CMC) portion of studies that support investigational new drug (IND) applications (1, 2). Desirable attributes of a CHO cell line include its ability to produce high titers of biotherapeutic proteins facilitate quick recoveries and selection processes maintain phenotypic and genetic stability throughout in-vitro aging of a culture. A CHO cell line also should be scalable to high-capacity culture…
eBook: Microbial Expression — The Right Choice for Large Peptides and Small Proteins
Although animal cell culture has dominated the biopharmaceutical industry for some years now, microbial expression remains important for producing proteins that don’t require posttranslational modifications — or only those that prokaryotic microbes can perform. It also offers an affordable option for antibody fragments and gene therapies. Microbes may be less fragile than animal cells, and they do require simpler media, but they present other challenges related to temperature management and oxygen transfer in culture. Wherever practical, bacterial expression is preferred…
Improving Bioprocess Expression Systems: A Clean Alternative to CRISPR/Cas9
Chinese hamster ovary (CHO) cells have emerged as a robust platform for bioprocessing serving both early and late-stage biotherapeutic drug supply. However, these cells and other hosts (e.g., HEK293), can be optimized for even greater potential through advanced gene editing. For example, when the endogenous glutamine synthetase (GS) gene is knocked out in CHO cells, a sixfold increase in high-producing cell lines is achieved (1). In another study, CHO with annexin A2 (ANXA2) and cathepsin gene (CTSD) knockouts were introduced…
eBook: Viral Vectors for Vaccines — A Virtual Conversation on Production and Analysis
Although today’s vaccines are safer, more effective, and more accessible than they were even 20 years ago, the emergence of new, complex pathogens has exposed limitations in traditional vaccine strategies. Viral vector vaccines (VVVs) hold great promise for confronting those now-intractable pathogens. Combining the best features of live-attenuated and DNA-vaccine approaches, these next-generation prophylactics seek to harness the infectivity of non- or low-immunogenicity viruses to shuttle antigen-encoding DNA from target pathogens into host cells. The resulting transduced cells then initiate…