Mammalian and microbial protein production platforms have been used for over 30 years to produce a number of successful biologic drugs, including monoclonal antibodies (MAbs), recombinant proteins, and therapeutic enzymes (1). Most biologics are produced by mammalian cell lines, with Chinese hamster ovary (CHO) cells being the most widely used. However, microbial cells also are used to express recombinant therapeutic proteins, and almost 30% of currently approved biologics are produced by Escherichia coli bacteria (2). With worldwide biologics sales >56…
Bioreactors
The Unican Concept: Engineering Dual Capability into Single-Use Vessels
Use of disposable bioreactors in the biopharmaceutical industry has increased gradually over the past several years in pilot, clinical, and production scale facilities (1–4). Reduced time to market in today’s drug industry has created a need for cost-effective development and production strategies as well as manufacturing flexibility. When compared with traditional stainless steel equipment, disposable bioreactor and mixing systems have smaller space requirements, are portable, and come presterilized to eliminate the need for preuse sterilization procedures such as steam-in-place (SIP).…
Process Development of Microbial Plasmid DNA: Fast-Tracking with Modular Single-Use Minibioreactors
There has been a rapid rise in the number of positive clinical outputs from clinical studies based on gene and cell therapies. This is in addition to the licensing of products such as GlaxoSmithKline’s Strimvelis ex-vivo stem-cell therapy for treatment of severe combined immunodeficiency caused by adenosine deaminase deficiency (ADA-SCID) in 2016 (1) — has led to an increase in demand for therapeutic vector manufacturing capabilities. Viral vectors are being used for an increasing range of conditions, including monogenetic conditions.…
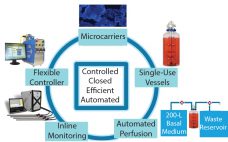
Difficult-to-Express Proteins: Resolving Bioprocessing Challenges with a Scalable Perfusion Bioreactor
Recent advances in protein engineering have identified new classes of complex biotherapeutics that challenge existing manufacturing platforms. These products have unique cell culture requirements that make them difficult to manufacture cost effectively. Industry standard bioprocessing platforms include large-scale (1,000–5,000 L) batch and fed-batch stirred-tank bioreactors. Historically, the powerhouse molecule of the biologics industry has been human IgG, which necessitates those large-scale platforms. Difficult-to-express proteins and other new modalities (including precision medicine and orphan drugs) have increased pressure on manufacturers to…
Aggregation from Shear Stress and Surface Interaction: Molecule-Specific or Universal Phenomenon?
Exposure to solid–liquid and air–water interfaces during production, freezing and thawing, shipment and storage of protein therapeutics may be a contributing factor in their degradation (e.g., aggregation, fragmentation) (1, 2). Surface exposure, particularly during manufacturing processes, often is accompanied by various degrees and durations of shear stresses originating from fluid flow and acting on proteins at interfaces. The magnitude and duration of shear rates depends on velocity gradients within each solution and varies significantly among manufacturing steps. On the low…
Platform Solutions for Cell Therapy Manufacturing
Advances in cell therapy have resulted in significant progress toward treating some widespread and difficult diseases, many of which represent unmet medical needs. For example, phase 3 clinical trials are already under way for therapies based on mesenchymal stem cells (MSCs), including therapies for graft-versus-host disease, acute myocardial ischemia, and chronic obstructive pulmonary disease (COPD) (1–3). Successful cell therapy treatments for such afflictions will be not only significant medical breakthroughs, but also in very high demand. However, their commercialization is…
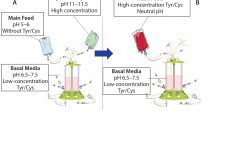
Simplification of Fed-Batch Processes with a Single-Feed Strategy
Chinese hamster ovary (CHO) cells commonly are used to produce recombinant proteins such as monoclonal antibodies (MAbs) for research, diagnostic, and therapeutic purposes. Culture processes typically rely on a fed-batch approach in which a basal medium enables initial cell growth. Concentrated feeds are used to prevent nutrient depletion, thereby extending culture duration and improving cell growth, viability, and protein titer. A neutral pH feed is desirable because culture pH should remain stable after feedings. The extremely low solubility of l-tyrosine…
Design and Performance of Single-Use, Stirred-Tank Bioreactors
Single-use components and systems have been incorporated into many bioprocesses as an alternative to cleanable, reusable systems. A wide range of publications have detailed the reasons for this trend toward a single-use approach. Justification in many cases comes from process-specific benefits such as increased manufacturing flexibility — especially for contract manufacturing organizations (CMOs) — enhanced sterility assurance, elimination of cleaning, reduced capital investment, faster processing times with increased productivity, faster start-up, and other benefits (1). One critical factor in the…
Continuous Cell Culture Operation at 2,000-L Scale
In the biopharmaceutical industry, continuous manufacturing is often cited as a method for increasing the productivity of bioprocesses (1). Compared with batch processing, it has the potential to enable production of more product within a smaller facility footprint — while improving product quality, particularly for sensitive and unstable molecules. Investigation into continuous methods is taking place for both upstream and downstream operations. For the full benefit of continuous processing to be realized, an argument has been made that cell culture,…
Performance Qualification of a Single-Use, Stirred-Tank Bioreactor with CHO-S Cell Culture
The increasing role and importance of cell culture in biophamaceutical manufacturing has led to considerable research and development (R&D) into bioreactor design and performance in recent years. As a result, a greater understanding of bioreactor fluid dynamics and critical physical parameters is now essential to maximize cell growth and productivity. Stirred-tank bioreactors are especially important in this development process because of their favorable properties in areas such as mixing efficiency and homogeneity, energy transfer, and scalability. The design and manufacture…