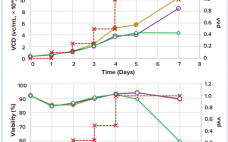
The recent outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) led to the development of different vaccine approaches worldwide to prevent the coronavirus disease 2019. The first registered vaccine on the market was the Sputnik V product based on two recombinant adenoviral vectors (Ad5 and Ad26). The product has received approval in 70 countries by several national and regional regulatory authorities, meanwhile. Though the availability of SARS-CoV-2 vaccines in most developed countries is not an issue any longer, other…
Bioreactors
Strategizing Scale-Up and Scale-Out for Cell Therapy Production
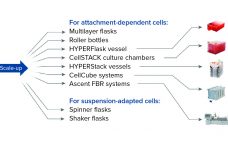
When considering strategies for expanding the number of cells being grown to support cell therapy development, companies often focus on decisions regarding scale-up and scale-out: increasing capacity either by using larger vessels to increase production volume or by implementing more units of the same vessel, respectively. Complete workflows often involve both. Figure 1 shows an example of scaling out from one to multiple cell culture flasks of the same dimension before transitioning to a larger format. Scale-out can be straightforward…
Ask the Expert: Facilitating Process Development Using a Microfluidic Perfusion Bioreactor
Suitable scale-down perfusion systems generally have been unavailable for process development (PD) activities. Some commercially available systems require daily media exchanges. No such system performs in a way that accurately represents large-scale perfusion, and none maintains sufficiently high cell densities. Kevin Lee (cofounder of Erbi Biosystems) joined BPI on 4 May 2021 to explain how his company’s Breez bioreactor system integrates all the functions of a stirred-tank reactor (STR) into a compact format that can facilitate PD, enabling one person…
High-Yield AAV Viral Vector Production in Corning Ascent Fixed-Bed Reactor System
Gene therapies hold great promise for one-time treatments that alleviate or cure certain genetic conditions. Recombinant adenoassociated viral (AAV) and lentiviral vectors have emerged as leading gene delivery methods for in vivo and ex vivo gene therapies, respectively. With more than 500 gene therapy clinical trials underway, the industry needs scalable, cost-efficient viral vector manufacturing. To address those needs, Corning developed the Ascent fixed-bed bioreactor system for scalable, high-density, adherent cell culture, including high-yield viral vector manufacturing. The Ascent fixed-bed…
eBook: Bioreactor Sensors —
Inside the Dynamics of Cell Culture
Cell culture monitoring can fall into something like a “black box” conundrum. Efforts to measure key parameters such as pH, glucose, and even cell density require sampling and removal of the contents from a bioreactor. But that procedure can expose both a process and an operator to contamination risks. Emerging bioreactor sensors are designed to address some of those challenges, but the rapid adoption of single-use technologies and the rise of perfusion cell culture have presented obstacles to their implementation.…
Rapid Development of Viral Vector Production Processes: Iterative Parameter Optimization
With recent developments and successes in cell and gene therapy, the biopharmaceutical industry is facing increased demand for safe and efficient delivery systems (1). Viral vectors, including adenoviruses (AV), adenoassociated viruses (AAV), and lentiviruses (LV), are among the most common delivery agents because they infect mammalian cells efficiently. Suspension cultures have become a popular choice for robust and scalable viral manufacturing systems. Using stable cell lines that integrate all or part of the viral production elements adds further benefits by…
Bioreactor Automation Driven by Real-Time Sensing: Enhancing Productivity Through Accurate, Efficient Glucose Control
In the quest for improved quality and productivity in drug manufacturing, the industry is moving toward increasing use of bioreactor systems with real-time integrated monitoring and advanced analytics that can enable automation, drive performance, and improve data-rich quality control. However, there are multiple options for sensors and technologies that monitor important cell-culture variables or critical process parameters (CPPs). Furthermore, cell culture vessels can be disposable single-use bioreactors (SUB) or reusable glass or stainless-steel models. They can operate in stirred tanks,…
Updating the Economics of Biologics Manufacturing with 5,000-L Single-Use Bioreactors: A Paradigm Shift
Single-use technologies enable a flexibility and modularity effectively unattainable with more traditional stainless-steel technologies, particularly in upstream bioprocesses. Single-use bioreactors up to 2,000 L are employed largely in preclinical- and clinical-stage bioprocesses to leverage this flexibility. As products reach commercial maturity, scales larger than 2,000 L frequently become desirable to take advantage of economies of scale. With the typical upper limit of single-use bioreactors at 2,000 L, this has traditionally meant transfer to stainless-steel systems. The introduction of the Thermo…
Dissolved Oxygen Control Tuning for Cell Culture Applications
Proper tuning of dissolved oxygen (DO) controller proportional integral (PI) values is essential for optimal cell culture performance in a bioreactor. When DO-PI values are optimized, gas flows are smoothed, and foaming and cell stress are reduced. Traditionally, this tuning has been performed by using nitrogen gas to purge oxygen from a test solution, thus simulating oxygen demand. That method has several drawbacks, however. First, nitrogen gassing cannot simulate the high demands of high-density fermentation. Second, nitrogen competes with other…
Retrofitting Your Bioreactor to Enhance Stirring Processes: Replacing Old Agitators with State-of-the-Art Magnetic Mixing Technology
A key challenge for companies involved in drug development is to meet the highest standards of sterile design and reliability. In this context, magnetic mixers offers many advantages for aseptic stirring processes compared to mechanically sealed agitators. ZETA not only supplies magnetic agitators for new bioreactors, but also supports its customers through the entire retrofitting process, from feasibility studies at the beginning to full process qualification and validation at the end. “Combat the risk of batch contamination in bioreactors and…