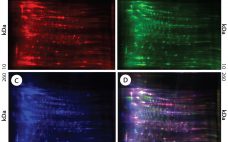
Using Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin…
Assays
Development of a Host-Cell Protein Platform Assay for a Chinese Hamster Ovary Cell Line
The Chinese hamster ovary (CHO) cell line is the most prevalent biopharmaceutical expression system and has been proven safe for commercial production of protein therapeutics (1). However, even after multiple purification steps, biopharmaceuticals contain residual host-cell protein (HCP) impurities that pose a potential safety risk to patients (2). Health authorities demand close monitoring of HCP impurities and require sensitive analytical methods with high coverage: the ability to detect a broad range of HCP impurities (3, 4). Polyclonal sandwich immunoassays are…
HCP Antigens and Antibodies from Different CHO Cell Lines
Cell lines derived from Chinese hamster ovary (CHO) cells are widely used in therapeutic protein production because they can perform human-compatible posttranslational modifications, they are easy to use for manufacturing, and they do not propagate most human pathogenic viruses (1, 2). Expressed therapeutic proteins are secreted into CHO culture supernatant along with impurities originating from the host cells themselves. Such host cell proteins (HCPs) are important contaminants for monitoring because they directly affect drug quality, safety, and efficacy. HCPs are…
High-Throughput Methods Evaluation: Impurities Determination During Upstream and Downstream In-Process Development
Getting biologic drugs through development and into clinical proof-of-concept studies quickly and efficiently is critical for success in the biopharmaceutical industry. Implementing high-throughput approaches to both upstream and downstream process development is increasingly helping companies stay competitive. Innovative and highthroughput analytical technologies are needed to support rapid process development. The study reported herein focuses on innovative immunoassay platforms for impurity-removal monitoring of both host-cell proteins (HCPs) and leached protein A. HCPs come from host cells during cell culture production. Their…
The Case for a Standardized Assay to Test Suitability of Single-Use Systems in Cell Culture Applications
Increased commercial use of single-use systems (SUS) for large-scale biopharmaceutical production creates the need for consensus on industry best practices and standards for materials in SUS components. End users and suppliers are beginning to develop a shared vision of industry needs in such areas (1, 2). For example, highly visible efforts to harmonize extractables testing include contributions from groups such as the BioPhorum Operations Group (BPOG), Bio-Process Systems Alliance (BPSA), Parenteral Drug Association (PDA), ASTM, and ISPE. In addition to…
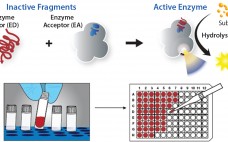
Accelerating Biologic and Biosimilar Drug Development: Ready-to-Use, Cell-Based Assays for Potency and Lot-Release Testing
With the drug industry’s expanding emphasis on biologics, the need for robust cell-based assays has grown at all stages of development. Requirements for efficacy, quality, and potency testing often demand a complex set of bioassays and/or cell-based assays for new therapeutics or biosimilars. Developers of the latter have found this need for cell-based assays to be particularly challenging. Commercially available, ready-to-use cell-based assays provide a robust functional response from specific therapeutic targets. They can significantly shorten assay development time while…
Assessing Similarity with Parallel-Line and Parallel-Curve Models: Implementing the USP Development/Validation Approach to a Relative Potency Assay
Potency is a critical quality attribute to support development and release of biopharmaceutical products. Researchers assess most protein-drug potencies using biological assays (such as cell-based assays), which mimic a product’s known mechanism of action or binding assays (if the only known mechanism of action is a drug binding to its target or if a drug is in early phases development). Potency denotes an important feature of complex biologics: their biological activity produced as a direct result of the molecule’s tertiary/quaternary…
Reference Standards for Therapeutic Proteins: Current Regulatory and Scientific Best Practices
Sponsors developing and manufacturing protein therapeutic products use a variety of analytical tests (e.g., cell-based potency and chromatographic assays) to assess quality attributes of their active ingredients and drug products. Those tests are used to assess product quality in a number of activities, including characterization, comparability, lot release, and confirmation product quality and stability. Reference standards play a critical role in calibrating and confirming the suitability of such tests and in helping analysts to draw scientifically sound conclusions from data…
Nucleic Acid Impurity Reduction in Viral Vaccine Manufacturing
Commercial-scale viral vaccine manufacturing requires production of large quantities of virus as an antigenic source. To deliver those quantities, a number of systems are used for viral replication based on mammalian, avian, or insect cells. To overcome the inherent limitations in production outputs with serial propagation of cells, mammalian cells can be immortalized, which increases the number of times they can divide in culture. Modifications that immortalize cells are typically accomplished through mechanisms similar to those converting normal cells to…
Ready-to-Use Cryopreserved Primary Cells
Abiological measurement of drug activity is perhaps the most critical step in the series of tests required for product release both for clinical trials and the market. This evaluation plays an important role in the stability assessment of drug candidates. According to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Q6B document, measurements of biological activity can be performed in defined animal models that demonstrate a measurable physiological change in response to…