Virus filtration is a vital part of the viral clearance strategy in bioprocesses. In filtration, size exclusion mechanisms are orthogonal to other inactivation or removal techniques and target the physical dimensions of the virus to achieve a high degree of virus reduction. Any flow or pressure interruption during filtration has been shown to increase the risk of virus passage. This application note reviews the PegasusTM Prime virus removal filters and demonstrates robust, high viral clearance in the presence of prolonged…
Sponsored Content
Special Report: A Strategy for Cost-Effective Capture Using Agarose-Based Protein A Resins
It is well recognized that the cost of Protein A resins is substantial. If a developmental monoclonal antibody (MAb) makes it to marketing approval and manufacturing, the high cost of purification using a Protein A resin is amortized over a large number of purification cycles, and the contribution to cost of goods is reduced to acceptable levels. However, a high percentage of clinical projects will fail, and the Protein A resin will be used only for a small number of…
Reveal Information That Gives Insights: New Approaches to Sub-Visible Particle Characterization
This webcast features: Josefina Nilsson, Head of EM Services, and, Gustaf Kylberg, Product Manager – MiniTEM, Vironova Sub-visible particle characterization is essential when comparing sample quality after different purification steps or for the understanding of product stability. Analysis performed with MiniTEM provides both morphology and accurate quantitative data that can help speed up process and formulation development. In this webinar you will learn: How MiniTEM automatically images, detects and classifies a large number of particles resulting in statistically significant and…
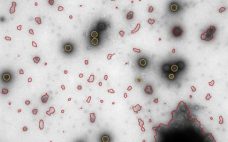
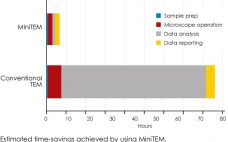
MiniTEM speeds up ‘time-to-insight’: automated purity analysis of two different Adenovirus samples
MiniTEMâ„¢ is a low-voltage transmission electron microscope system designed for nanoparticle characterization. The high-quality images it acquires reveal particle morphologies that can be transformed into accurate metrics. A metric for sample purity based on the ratio of the total area of debris to that of intact Adenovirus particles is presented in this study. The automated analysis that MiniTEM offers encompasses a far larger number of particles compared with studies performed manually using conventional electron microscopy, resulting in accurate and statistically…
A summary of how to develop a cell therapy from concept to market authorization
Cell therapies offer new opportunities for the treatment of disease and injury but considerable infrastructure and a complex range of activities and expertise are required to translate a promising cell therapy product into clinical use. Suppliers, developers, regulators, and care givers need to interact cooperatively in order to bring therapies to patients in the most efficient, safe, and economically viable way and GE Healthcare is working with the Karolinska University Hospital to investigate more effective routes for bringing potential cell…
An Example of Evaluation of protein A Multicolumn Processes for the Capture of Large-Volume, High Concentration Bioreactors, Based on BioSC® Predict Optimization Software
The present paper provides some recent data regarding the optimization of BioSC® processes using three commercially available protein A resins, based on the BioSC® Predict software. A rapid description of the first steps to develop a multi-column process guided by simulation is provided to avoid generating unrealistic conclusions. Other approaches could however be applied depending on the objectives targeted. Examples of multicolumn systems based on three commercially available protein A resins are also provided, for the purification of a 15,000-L…
Improving Single-Use Bioreactor Design and Process Development: New Research Towards Intensifying Seed-Train and Scale-Up Methods Using 5:1 Turn-Down
This webcast features: Surendra Balekai, Senior Global Product Manager, Thermo Fisher Scientific Bioproduction Operating bioreactor vessels at low working volumes (low turn-down ratio) is often desirable but brings about challenges in regard to mixing, mass transfer, and process control. Research done toward optimizing cell culture has provided methods to improve performance and control when operating under these conditions. Implementing bottom heat exchange, making changes to impeller angle and height, taking advantage of the unique Thermo Fisher drilled-hole sparge design, and…
Top 5 Considerations When Looking to Outsource to a Biomanufacturing Partner
This webcast features: Paul Jorjorian, Director, Global Technology Transfer, Patheon Biopharmaceutical companies are facing increasing constraints when developing and manufacturing their large molecule drugs. Development labs and manufacturing suites have limited capacity making it nearly impossible to right size internally for uncertain forecasts. As a result, selection of the right biomanufacturing partner has never been more important – for small and mid-size biopharma companies alike. There are an ever-increasing number of options and many criteria to consider during the vendor…
Practical Aspects of Implementing Continuous Bioprocessing
This webcast features: Dr. Marc Bisschops, Principal Scientist, and Dr. Mark Schofield, Principal Engineer, R&D, Pall Life Sciences The next evolution in the biopharmaceutical industry is the widespread adoption of integrated continuous bioprocessing for biologics manufacturing. The key to its success, however, is the availability of novel upstream and downstream technologies that will not only reduce facility footprint, capital expenses, and product cost of goods, but will also increase process productivity, flexibility, and further facilitate the utilization of single-use and/or…
How to Boost Profits with Single-Use Powder Transfer in Biopharmaceuticals Manufacturing
For many years, media and buffer ingredients used in powder form were transferred from stock containers using open scoops, weighed and mixed in buckets or open-top bags, and then carried in and dumped from those buckets or open-top bags directly into production vessels—based on the premise that sterility wasn’t required at that early stage of manufacturing. While much of this process was often carried out in a separate room from the production line to contain airborne contaminants, final transfer to…