The quality of pharmaceutical products is the top priority for both biopharmaceutical companies and regulators. To ensure consistently high product quality and improve the efficiency of manufacturing and regulation, the US FDA introduced quality by design (QbD) to the pharmaceutical industry in its 2002 Pharmaceutical CGMP initiative, Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach (1). Since the introduction of QbD, the FDA has taken multiple measures to promote industry-wide implementation, but the industry’s response to QbD has been…
Sponsored Content
The Many Strengths of One Film: Fortem™ Single-Use Platform Film Built for Bioprocess
Single-use technology has been transforming the modern biomanufacturing workflow. You can now find single-use bags at every stage of bioprocessing: bioreactor and mixing systems, harvest and collection, purification, liquid and powder storage, and transportation. The Strength of Simplicity: One Film for Bioprocessing At GE, we’re introducing one film for our entire portfolio of single-use systems. It’s named Fortem after the Latin word for strong — and aptly so because it is strong in many ways. It was born of customer collaboration, supplier…
MabPlex: Custom, Flexible, and Reliable Contract Development and Manufacturing
MabPlex provides world-class solutions to biopharmaceutical clients around the world. Our company is based on the quality by design (QbD) principle, from site construction to manufacturing and delivery of our clients’ biologic drugs. With an expert team adept in both chemistry and biology, MabPlex provides global contract development and manufacturing organization (CDMO) services in biopharmaceuticals. Capabilities span the scope from monoclonal antibodies (MAbs) and fusion proteins to the most complicated antibody–drug conjugates (ADCs). Our 275,000-ft² manufacturing space offers flexibility and…
Rentschler: A World-Class Biopharmaceutical CDMO
Rentschler Biotechnologie GmbH, located in Laupheim, Germany, is a leading biopharmaceutical contract development and manufacturing organization (CDMO) for bioprocess development and manufacturing of biopharmaceuticals and an experienced specialist in mammalian cell culture. The company’s clients include innovative biotech companies and major pharmaceutical companies around the world. Many years of experience and excellence in finding solutions as well as certified quality management and advanced technologies ensure Rentschler’s high-quality standards. Rentschler Biotechnologie is a family-owned company employing approximately 700 people. Full-Service Provider…
Biopharmaceutical Development and GMP Manufacturing: Preclinical to Commercial Supply
Richter-Helm is a Hamburg, Germany–based contract development and manufacturing company with a proven 25-year track record, specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines. Our seasoned, 160-strong team supports you with process development, supply of products for clinical…
The Microbial CMO: Process Development and Manufacturing of Biologics
Wacker Biotech is “The Microbial CMO” — the partner of choice for contract manufacturing of therapeutic proteins using microbial hosts. Our service portfolio covers molecular biology, process and analytical development, and the GMP production of biologics for clinical trials and commercial supply. Founded in 1999 as a spin-off from the Hans-Knöll Institute in Jena, we are a 100% subsidiary of Wacker Chemie AG since 2005. The sites in Jena and Halle provide a complete range of services for the development…
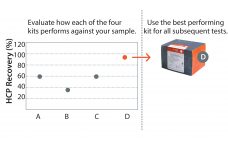
Is Your HCP Assay Fit for Purpose?
Removal of host cell protein (HCP) impurities in drug substances is critical in the manufacture of highquality drug products and for patient safety. A key requirement is a thorough analysis of HCP contaminants, which may vary considerably depending on cell line, media, and process parameters. How well a particular HCP assay recognizes all HCPs will depend on how well its antibodies match the actual HCP composition, their abundance, and affinities for each HCP. Accordingly, various commercial generic HCP ELISAs may…
Dissolved Oxygen Quantification in a DO-Sensitive Product: Study of DO Values at Laboratory and Industrial Scales
Sulfur is an essential component of all living cells. In plants and animals, the amino acids cysteine and methionine contain most of the sulfur, and the element is present in all polypeptides, proteins, and enzymes that contain these amino acids. Sulfur-containing compounds often show different biological activities and provide important functions in pharmaceutical industry applications. Sulfonamides, thioethers, sulfones, and penicillins are the most common scaffolds in sulfur-containing drugs. Sulfonamide drugs were the first antibiotics to be used systemically, and they…
Nonaffinity-Based IgM Purification Platform
The biological properties of IgM antibodies make them very effective vehicles for in vitro diagnostics and therapeutics. However, purification of IgM antibodies is far more complex than that for IgG antibodies. Furthermore, affinity chromatography is not ideal for purifying IgMs due to the required elution conditions. Here we propose a nonaffinity-based platform for IgM purification. This strategy utilizes a cation exchange (CEX) media, Nuvia™ S, for capture, and a mixed-mode media, CHT™, for polish purification. It is suitable for purification…
Effective Viral Clearance in mAb Purification Using TOYOPEARL® Resins
Impurities such as viruses are typically present during the manufacturing of biological drugs. The viruses may be present in the unprocessed material from the upstream collected harvest, such as released from cell culture media (endogenous) or accidentally introduced during processing (adventitious). Viral clearance is required by the FDA and international regulatory bodies as specified in the ICH Q5A documents. In this study we evaluated two chromatography steps in the purification of a monoclonal antibody (MAb) for viral clearance. Evaluation was…