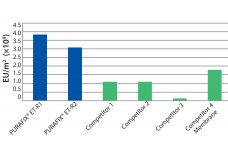
What factors impact peptone variability? Variability can be linked to at least two critical elements: the biological raw materials and the production site. Raw materials can vary by species of animal or plant sourced, type of components (specific animal tissues as opposed to standardized materials), and geographical origins. Feeding practices also can cause variability. Within a single manufacturer, interbatch variability can be linked to the condition and age of a production site, its level of automation and sophistication, optimized production…
Sponsored Content
How Clean Is Your BSA?
Bovine serum albumin (BSA) is a critical component of many biotechnology and biochemistry systems. BSA is used in a variety of applications, including diagnostics, veterinary medical products, vaccine manufacturing, mammalian cell growth for biopharmaceutical production, and topical and ex vivo medical device applications. The impact of BSA on the performance of these systems is often overlooked and is identified as a possible root cause of variability only after extensive testing and elimination of other system components. There are many different…
Unlock Pichia: High-Level Methanol-Free Protein Production in Pichia pastoris
Pichia pastoris (Komagataella phaffii) is recognized as a highly competitive expression system for fast and economic production of recombinant proteins. Pichia offers many advantages. It is an established (FDA and EMA approved), safe (generally recognized as safe, GRAS), and highly competitive expression host with strong and effective secretory capacities often resulting in double-digit grams-per-liter product levels in culture supernatant. It combines advantageous properties of prokaryotes and mammalian cells. On one hand, Pichia cultures grow fast and can reach high cell…
Genderless Sterile Connectors: How Single-Use Connectors Can Increase Efficiency, Saving You Time and Money
Standardizing equipment in biopharmaceutical production streamlines operations, saving end users time and money. An area ripe for standardization is the use of genderless connections, those in which the connector halves are identical in design. Historically, single-use connections have involved male and female halves, but using two different parts can create several inefficiencies and complications, including Increased ordering complexity Greater risk of specifying the wrong system or connection, resulting in downtime and last-minute adjustments Longer lead times Increased stocking requirements. Login…
The Forgotten Immunoglobulin LigaTrap™ Technologies and Advancements in IgM Purification
It’s been over 30 years since the identification and commercialization of protein A. Since its inception, protein A has served as the pinnacle purification platform for the immunoglobulin G (IgG) subclass of antibodies, both monoclonal and polyclonal forms. The introduction of protein A paved the way for researchers and large pharmaceutical manufacturers to develop and commercialize IgG-based therapeutics at both small and large scales, provided by its relative ease of use and well-characterized chromatographic attributes. Unfortunately, the gold standard affinity…
The Experts in Noninvasive Liquid Flow Measurement Via the Ultrasound Transit Time Method
Germany-based em-tec GmbH has been developing and manufacturing products and components for the medical and bioprocessing industry for decades. With many years of experience, em-tec has become known as a strong partner for consulting, development, and production focusing on noninvasive flow measurement systems using the ultrasound transit time principle. The long-established and medically certified bidirectional clamp-on flow measurement system for liquids has been adapted for the special needs of the good manufacturing practice (GMP)–oriented bioprocessing industry. The Flow Measurement System for…
Endotoxin Removal in a New Scalable Dimension
Endotoxins are degradation products from dying gram-negative bacteria and complex aggregates of acidic lipopolysaccharides (LPS). Each is composed of lipophilic lipids and hydrophilic polysaccharides. In humans, endotoxins can cause immune responses such as fever (pyrogenic threshold is approximately 0.1 ng/kg body weight). Unlike bacteria themselves, endotoxins are extremely heat and pH stable and therefore withstand sterilization methods. During protein purification, the reduction of endotoxins is one of the most important and difficult steps. It often includes complex purification strategies (e.g.,…
Bioburden: Current Innovations and Practices to Address Microbial Contamination in Downstream Bioprocessing
Bioburden control is an area of serious concern for both manufacturers of biologicals and suppliers to the industry. This white paper considers some of the risks related to downstream processing and presents recent developments by suppliers that help manufacturers mitigate these risks. Topics covered include improvements in raw material quality, equipment design, chromatography resin properties, and ways of working. Challenges specifically related to the sanitization of protein A chromatography resins also are discussed. Bioburden Control in Biopharmaceutical Production The risk…
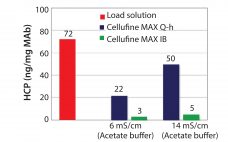
A Novel Cellufine Mixed-Mode Resin: Cellufine™ MAX IB for Polishing of Monoclonal Antibodies
Cellulose is well-known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. Additionally, cellulose particles have unique pore-size characteristics appropriate for the chromatography of biopharmaceuticals. Mixed-mode chromatography resins are well known to have unique selectivity differences from traditional IEX or HIC resins. Cellufine™ MAX IB, a novel cellulose-based mixed-mode resin, has polyallyl amine partially modified with butyl groups ligand (Figure 1). The resin is used in flow-through mode after a protein A step in…
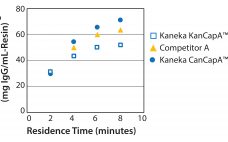
KanCapA™ 3G: An Innovative Protein A Resin for MAb Manufacturing
Recent improvements in monoclonal antibody (MAb) upstream process technologies have led to increased product titers (from 5 to 10 g/L) and a corresponding change in impurity levels. To yield highly pure MAb drugs from such high-titer feedstocks, new, robust, and cost-effective purification processes need to be developed to follow this upward trend. KANEKA has developed KANEKA KanCapA™ 3G, an innovative protein A resin that demonstrates higher binding capacities and allows milder elution conditions than well-known high-binding capacity agarose based resin.…