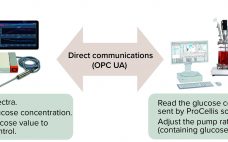
The process analytical technology (PAT) and quality by design (QbD) guidelines promoted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support the idea that quality cannot be “tested into” a biologic product but must instead be part of its process design. Seamless integration of analytical data with bioprocess monitoring and control is crucial to understanding a process and overcoming manufacturing challenges that arise in the course of development. Monitoring of product quality attributes (PQAs)…
Sponsored Content
Considerations for Developing Inhaled and Nasally Delivered Biologics
Inhalable drug formulations show much promise for improving biopharmaceutical delivery. But as Ashleigh Wake (director of business development at Intertek) explained to Ask the Expert attendees in September 2021, such formulations necessitate development of complex quality control (QC) analytical methods. Wake highlighted special considerations for characterizing and controlling the stability and potency of inhaled and nasally delivered biologics. Wake’s Presentation Wake emphasized that nasal and inhaled biological products necessitate QC strategies to control both a protein product and its delivery…
Human Umbilical Cord-derived Mesenchymal Stem Cell Production in Corning® HYPERStack® 36-layer Cell Culture Vessels
As more clinical trials are evaluating MSC-based therapies, the demand for more pertinent adherent scale-up tools is likely to increase. Corning® HYPERStack® 36-layer cell culture vessels offer a closed system solution for scaling up large quantities of quality, human umbilical cord-derived MSCs. Most importantly, the MSCs expanded in the HYPERStack 36-layer vessel expressed high percentages of CD90, CD105, and CD73, characteristic of MSC quality. The ability to grow large quantities of human umbilical cord derived MSCs with high viability and…
Whitepaper: Intensifying Upstream Processing – Implications for Media Management
Process intensification offers many benefits but implementing PI can introduce an unexpected challenge of managing larger media volumes. This whitepaper helps ensure your implementation strategy accounts for the increased media volume associated with process intensification. What You Will Learn Gain the tools and knowledge needed to confidently assess intensification options with a focus on media management for new or existing facilities. Follow the media journey from prep to use, exploring potential logistical pitfalls in the management of increased media volumes…
What’s new in AAV manufacturing? 3 takeaways from Thermo Fisher Scientific
From virtual regulatory inspections to optimization of scalable AAV production, the gene and cell therapy fields continued to innovate and evolve throughout the pandemic. Thermo Fisher Scientific hosted an interactive workshop highlighting some of these developments, including a new AAV production system, a custom media platform for viral production cell lines, and changes to the regulatory process. Read on to get a taste of those insights. A Helper-Free AAV Production System Adeno-associated viruses (AAVs) are among the most promising gene…
Practical Approaches to Metals Analysis of Cell Culture Media & Impact on Therapeutic Protein Production Using ICP-MS
This webcast features: Dr. Adil Mohammad, Staff Fellow, US FDA, Dr. Chikkathur Madhavarao, Biologist, US FDA, Robert Thomas, CSci, CChem, FRSC, Principal Consultant, Scientific Solutions Biotechnology products (biologics) are often produced from mammalian cells grown in large-scale bioreactors. The dynamic environment within the bioreactor is comprised of growth media along with cells, proteins, nutrients of organic and inorganic origin, metabolic waste products and metal ions. Metal ions can act as enzyme cofactors and can directly influence the kinetics of biochemical…
Gain Control of Culture Conditions: Technology for Sustained Delivery of Recombinant Proteins
Growth factors, added at the precise time and concentration to in vitro cultures are essential to control cell proliferation and differentiation. When added to a culture vessel, however, the concentrations of these signaling proteins rapidly decline, altering both levels of individual growth factors and the ratio of factors for cell signaling. When growth factors are replenished by exchanging the medium, concentrations peak, resulting in an ebb and flow of growth factor levels resulting in mixed signaling that can lead the…
A Frost & Sullivan Virtual Think Tank: 3M™ Polisher ST Beta Testing Series
One step can change everything: Replace your flow-through AEX column with a novel single-use polishing solution 3M™ Polisher ST single use polishing solution – from lab to manufacturing scale Frost & Sullivan recently invited a panel of bioprocessing industry leaders and key opinion leaders to participate in a Virtual Think Tank (VTT) Early Access series – a new and unique thought leadership forum. Each VTT panel, comprised of professionals from top pharmaceutical companies, contract development and manufacturing organizations (CDMOs) and…
Build vs. Buy: A Critical Calculation for Cell Therapy Innovators
Cell therapy is proving to be one of the most promising advanced modalities, representing a significant step forward in the treatment of a wide range of challenging diseases and conditions. As a cell therapy candidate advances from discovery through clinical development and ultimately to commercialization, foundational decisions must be made by the product sponsor that will impact both scientific and commercial success. One of the biggest decisions is whether to build a manufacturing facility or outsource to a contract development…
Adenovirus Vector Manufacturing Platform Using CIMmultus QA
This webcast features: Hana Jug, Project Manager in Process Development for Viral Vectors and Vaccines, BIA Separations Downstream processing remains one of the main bottlenecks in Adenoviral vector manufacturing. At BIA Separations, a Sartorius company, we offer a platform for purification of Adenoviral vaccines using market leading CIM® monolithic chromatographic columns and an analytical toolbox for process monitoring in Adenoviral vaccine production. Simplified purification of Adenovirus consists of typical downstream steps, including combined lysis, clarification, TFF and a chromatographic capture…