The generation of human induced pluripotent stem cells (iPSCs) via reprogramming technology represents a major breakthrough in personalized medicine and the treatment of degenerative diseases. This is mainly because the iPSCs can be expanded in culture and then differentiated into specialized cell types that can be used for clinical applications. Patient-derived iPSCs can be used to model human genetic diseases, produce clinically relevant differentiated cells that display disease pathogenesis, or generate specialized cells through directed differentiation process for autologous cell…
Sponsored Content
The Multi-Mode Mimetic Ligand Library: A New Tool for Rapid Development of Downstream Processes
Recent developments in downstream processing of biomolecules — including continuous processing, bind–elute affinity capture, and flow-through polishing steps — have increased the need for greater selectivity from chromatography adsorbents. This has led to the introduction of a new generation of adsorbents: so-called “mixed-mode” or multimodal ligands. They provide greater selectivity and tolerance to process buffer composition than either ionexchange chromatography (IEC) or hydrophobic-interaction chromatography (HIC) alone can provide. Learn more in this Special Report from Steve Burton, Chief Executive Officer…
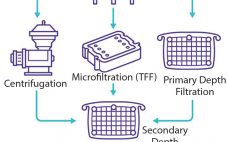
Filter-Based Clarification of Viral Vaccines and Vectors
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
Ensuring the Integrity of Single-Use Containers: Providing Robustness, Science, and Helium-Based Technology with a Detection Limit of 2 ÎĽm
Identifying the greatest defect size, both for liquid leaks and microbial ingress, is a fundamental step toward protecting the integrity of single-use systems (SUS) under real process conditions. Integrity testing of such systems may become a prerequisite in the future because they are used in the most critical process steps, with detection limits correlating to liquid leaks and microbial ingress. Such testing guarantees the sterility of drug substances and drug products packaged in single-use systems and, therefore, enhance patient safety.…
Scaling Up? No Problem with New 80cm Diameter Pre-packed OPUS Columns
This webcast features: Fletcher Malcom, Director of Product Management, Downstream Technologies, Repligen The technical and economic benefits of ready-to-use, pre-packed chromatography columns have been proven and documented. The question remains, how large can we go? This presentation will show how the unique design of the new 80cm pre-packed OPUS column delivers chromatographic performance, and how usage can result in substantial cost and time savings for clinical and commercial scale manufacturing.
Ask the Expert: Integration of Cell Line, Process, and Analytical Technologies: Speeding Development and Clinical Supply of Emerging Therapy Products
Chinese hamster ovary (CHO) cells are being pushed to their productivity limits in drug development and biomanufacturing. Biopharmaceutical companies working on promising new therapies increasingly struggle with development challenges such as weak protein expression, low-yield purification steps, and poorly optimized analytical techniques. To address those challenges, innovators need robust cell-expression platforms and advanced process, analytical, and biomanufacturing technologies. Since 2012, KBI Biopharma has performed development and/or manufacturing services using more than 20 different cell lines generated by Selexis SA. In…
Removal of Isoagglutinins from IVIG and Plasma Using Affinity Chromatography
Antibody-mediated haemolysis is a hard-to-predict phenomenon with potentially severe consequences. It is mediated by naturally-occurring anti-A and anti-B immunoglobulin isoagglutinins, which are present in plasma, blood, and several derived products, including IVIG produced by plasma fractionation. Prometic Bioseparations have developed an affinity chromatography resin for the removal of isoagglutinins from plasma and plasma derived products, such as IVIG. The resins, IsoClear A and IsoClear B, can clear isoagglutinins from a titre of 1/32 down to negative agglutination using a load…
Removal of Endotoxins – From Bench to Process Scale
Endotoxins or lipopolysaccharides (LPS) are highly toxic components of the cell wall of Gram-negative bacteria, which are often present in significant amounts in bacterial cell expression systems such as E.coli. A number of methods have been adopted for the removal of endotoxin based on adsorption, in particular ion exchange chromatography. Although downstream processing can significantly reduce endotoxin levels in the product, efficient and cost effective removal of residual endotoxin from biopharmaceutical preparations remains a challenge. Prometic Bioseparations have developed an…
Advantages of Fluoropolymer Single-Use Systems
This webcast features: Eric Isberg, Director of Life Sciences, Entegris There remain significant performance risks restricting the use of disposables in bioprocessing. These factors include bag breakage, which is particularly evident in cold temperature applications; concerns around E&L and material purity; and use of materials which are incompatible with the process itself. Improved plastics are required to meet existing and future process needs; fluoropolymers appear to be very promising to achieve these goals. Fluoropolymers’ unmatched characteristics are inherent to their…
BPI West TV 2018: Dr Joswin Kattoor, Product Manager Pharma, DPL
At BioProcess International West (March 2018) Maribel Rios, Managing Director at BioProcess International, caught up with Dr Joswin Kattoor, Product Manager Pharma at DPL (Dr Paul Lohmann) for BPI TV. DPL manufacture a wide range of organic and inorganic salts, producing 400 products. According to Dr Kattoor, their biopharma customers are most often looking for speed to market – “raising a new product from initial discovery, to manufacturing, to product launch without failing in a clinical trial.”Salts play various roles…