Chinese hamster ovary (CHO) cells have been used in biomanufacturing for decades because of their robust capacity to express a range of proteins, such as therapeutic enzymes and monoclonal antibodies (MAbs) at titers measured in multiple grams per liter of culture. Within the available suite of CHO cell lines, the glutamine synthetase knockout (GS-KO) selection system provides industry-leading speed to the identification of high-producing clones for use in biomanufacturing. The GS-KO selection system allows for identification of multiple-gram/L clones in…
Sponsored Content
Automating Workflow Solutions, Not Instruments: Engineering Validated Processes for Biologics Screening
This webcast features: Brian Bordeau, Business Development, Global Advanced Workflows and Engineered Solutions, FortĂ©Bio Automation can be a lifeline for scientists working in the discovery and development of biologics. The prospects for increasing sample throughput, reducing operator time, and streamlining data audit trails are alluring. However, at the same time, introducing automation can be a frightening and confusing project, with efforts often falling short of expectations for lack of bigger picture thinking. The Advanced Workflow Engineering Solutions (AWES) team at…
Clearing the Way for Viral Clearance
This webcast features: David Cetlin, CEO, MockV Solutions and John Li, Staff Scientist, Thermo Fisher Scientific To determine the viral clearance efficacy of biomanufacturing steps, mammalian viruses are “spiked” into in-process solutions, processed and analysed for reduction. Due to the infectious nature of these live viruses, “spiking studies” are typically conducted in specialized BSL-2 facilities. The costs and logistics associated limit viral clearance analysis during process development and characterization. To overcome this challenge, a non-infectious Minute Virus of Mice –…
Cell Culture Bioprocessing in Perfusion: Assessing Cell Retention Technologies
Upstream bioprocessing in perfusion mode holds great promise for industrial production of cells and biologics. In perfusion, fresh medium is added constantly to the bioreactor, and used medium is harvested while the cells are retained in the bioreactor. As a result, the composition of the cell culture medium stays quite constant during the process. This offers several advantages. In perfusion, higher cell densities can be reached than in batch and fed-batch processes, therefore enhancing volumetric productivity. Because medium composition can…
A Strategy to Remove Formulation Development from the Critical Path During Biologics Development
Biopharmaceuticals tend to be highly unstable. Therefore, as the product development program progresses to phase 3 clinical stage, formulation development is required to ensure drug product quality and stability during manufacturing, storage and clinical administration. Also, formulation development is often on the critical path to successful IND and BLA fillings. During this Q&A, Dr. Jun Lu, Director, Analytical Development, discusses how Catalent Biologics utilizes automation, specifically the Uncle platform, during formulation development. Login and click View PDF to view the…
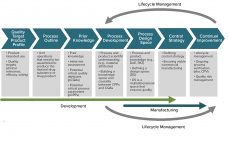
Step-wise strategy to address process characterization and late phase development – toward the definition of a standardized approach
Drivers for process characterization and late phase development include improving process understanding, enhancing process robustness, and assurance that the process delivers consistent product quality within all Proven Acceptable Ranges (PARs). Regulator’s expectations for biologic submissions include the application of statistical methods to improve the confidence of the PARs and knowledge of the design space for a process. Different approaches have been reported for process characterization but contain common elements including risk assessment, scale-down model qualification, and statistical design of experiments.…
Multi-Product Facility Realized by Modular Automation Platform
This webcast features: Dr. Burkhard Joksch, Product Manager, Bioprocess Automation at Sartorius Stedim Biotech GmbH, and Dr. Stuart Tindal, Product Manager, FlexAct® Platform at Sartorius Stedim Biotech GmbH Multi-product facilities need modular package units to realize fast change-over without reducing product quality and process performance. Smart modular package units such as the FlexAct® platform can run pre-qualified and pre-tested recipes, users can rapidly integrate Sartorius Stedim Biotech’s and other manufacturers’ single-use technology in bioprocess operations, achieving faster installations with reduced…
Visible Particulate Matter in Single-Use Bags: From Measurement to Prevention
Parenteral pharmaceuticals must be “essentially free” from visible particulate matter (1). In the production of biopharmaceuticals with single-use systems (SUS), biocompatibility requires controlling interactions between drug substances/products and SUS surfaces to ensure drug product quality and patient safety with regard to extractables/leachables and particulate matter. Any particulate matter stuck to fluid-contacting surfaces of process components could wash off and contaminate process fluids. Depending on system configuration, a final drug product could be at risk for particulate matter from SUS. Risk…
Is the QbD Toolbox Ready for Cell and Gene Therapies? Integrating Patient Outcomes into Manufacturing Cell and Gene Therapy Bioproducts
In their lifecycle development and manufacturing models, biotechnology products and the biopharmaceutical industry have been founded on principles originating from the pharmaceutical small-molecule industry. Such principles define clinical programs that establish risk benefits of a dosage and its delivery system on healthy individuals and patients. A company then develops a process to manufacture that product consistently over several years. Product quality attributes set through manufacturing controls are expected to ensure patient outcomes in terms of safety and efficacy and deliver…
ekko™: How Acoustics Cell Processing is Changing Cell and Gene Manufacturing
This presentation features: Kevin Lannon, Director of Global Sales, FloDesign Sonics With an ever-increasing number of cell and gene therapies approaching commercialization, the industry is looking for scalable manufacturing solutions. In order to truly see a step change in improvement, it is crucial for tool providers to design equipment specifically for the GMP environment and the unique needs of cell and gene processing. This presentation will outline: How acoustophoresis and the ekko™ platform is changing the cell processing paradigm Overview…