This webcast features: Ramsey Shanbaky, Associate Director, Bioprocess Applications, Repligen In-process measurement is the key to process monitoring and control. It can save time and money by helping you identify key aspects of your process in real-time. In this webcast, we will demonstrate how the CTech™ FlowVPX™ Slope Spectroscopy System, when directly integrated in one or more locations of a process stream, has helped reveal process characteristics that until now have been hidden due to the limitations of the most…
Sponsored Content
Facilitating Workforce Development: A case study on improving single-use training through vendor and end-user collaboration
Discover how Pall Corporation and Lonza collaborated to improve single-use technology training for operators using a blended approach to learning. This article presents: The importance of SUT training for operators. Why a blended approach ensures that operators get the training they need in the format that best suits their learning style. How collaboration between suppliers and biomanufacturers can shorten training program development timelines and increase the quality of training tools. How Pall and Lonza developed a digital training approach together.…
AAV Downstream Process and Product Characterization: Integrating Advanced Purification and Analytical Tools into the Workflow
The optimization of the downstream process for Adeno-associated virus (AAV) production with consistent quality depends on the ability to characterize critical quality attributes affecting potency, purity and safety of the final product. As the gene therapy field continues to push products through the clinical pipeline, an increasing need for efficient purification and analytical tools has become evident. In addition, the regulatory space has expanded in parallel to the use of AAV, driving the demand for simple and efficient assays to…
Security of Supply Risk Mitigation in HCP Monitoring
The most common method scientists use to monitor host cell protein (HCP) levels in biologics is the enzyme-linked immunosorbent assay (ELISA). For the reagent, security of supply is a regulatory requirement â â itâs a factor thatâs critical to consistently monitoring HCP during biologic production. In this webinar, we discuss why security of supply is important, the consequences of not having it, and how to mitigate this risk. Topics covered Why security of supply is critical to your HCP plans Consequences…
The Production and Application of Antibody Fragments
Antibodies are modular defense systems that identify and neutralize foreign objects like bacteria and viruses. In addition to oncology and inflammation, antibodies are now recognized as routine molecules in many therapeutic fields. Antibody fragments, in particular the Fabs, scFvs and VHH retain full antigen-binding capacity and superior properties for research, diagnostic and therapeutic applications. They have a smaller size that enables their binding to hidden epitopes not accessible to whole antibodies. In the context of therapeutic applications, a small molecular…
WACKERâs plug-and-play technology serves growing pDNA demand for cell and gene therapy
The global demand for nucleic acid-based gene therapies, novel vaccines and innovative therapeutic agents, including messenger RNA (mRNA) and viral vectors, is extremely high and projected to increase in the future. Plasmid DNA (pDNA) is the basis for all of these advanced therapies. Plasmid DNA can be used either directly for vaccines and gene therapies, or as starting material, e.g., to manufacture mRNA. The CDMO market for pDNA is expected to grow by 19 percent by 2025 (Global Viral Vector…
Chromatography in mRNA Production Workflow
Rapid response to global pandemics requires the manufacture of billions of vaccine doses within months. This short timeline must allow for design and testing of active ingredients, development of production and purification processes, clinical evaluations, regulatory filings, and manufacturing. Existing purification methods often have been adopted from laboratory-scale techniques to allow rapid implementation, and those have provided adequate product quality. But future mRNA development will require optimized production and purification processes. Chromatography has been a workhorse of biomanufacturing for decades,…
Purity By Design
Astrea Bioseparations has a well-established modular program to support customer projects from small to large scales with ligands, adsorbents, and chromatography columns that design purity into each process. Demand for increased productivity in biopharmaceutical manufacturing has placed new pressure on downstream purification operations. For recombinant proteins and monoclonal antibodies (MAbs), such pressure stems from significant gains in upstream productivity, particularly from high titers produced using increasingly efficient cell-culture systems. For viral vectors used in gene and gene-modified cell therapies and…
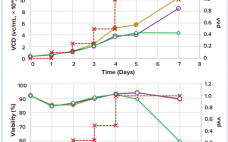
High-Yield Production of rAd26-S for Sputnik V Vaccine Component I: An Optimized Process in a Scalable Shaken Bioreactor
The recent outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) led to the development of different vaccine approaches worldwide to prevent the coronavirus disease 2019. The first registered vaccine on the market was the Sputnik V product based on two recombinant adenoviral vectors (Ad5 and Ad26). The product has received approval in 70 countries by several national and regional regulatory authorities, meanwhile. Though the availability of SARS-CoV-2 vaccines in most developed countries is not an issue any longer, other…
Tris, a Critical Raw Material: Improving the Quality and Consistency of Supply
ANGUS Life Sciences is the worldâs largest supplier of tromethamine buffers and the only manufacturer of the tris molecule based in the Western hemisphere. The company sells directly to biopharmaceutical customers and contract manufacturing organizations as well as to reprocessors who repackage the chemical or process it into different grades and derivatives. After recent expansions in both the United States and Germany, the company now boasts dual-source manufacturing capabilities for its highest-purity tris products and is confident about its ability…