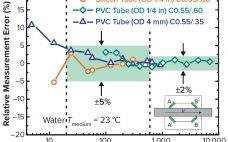
Single-use systems (SUSs) are becoming increasingly common in bioprocessing operations because of their low capital requirements and validation costs. As this trend continues to develop, pharmaceutical manufacturers are asking SUS manufacturers to provide assurance that their products comply with current good manufacturing practices (CGMPs) and do not alter drug products by exceeding established operating ranges. Certification of product cleanliness has become common for manufacturers of final packaging components such as vials and stoppers, but rarely are tubing products certified to…
Sponsored Content
Ask the Expert: Automated, Closed-Loop, Inline Monitoring of CAR T Cells in a Production Process
Jan Van Hauwermeiren (vice president of sales and marketing at Ovizio Imaging Systems) joined BPI for an Ask the Expert webinar on 29 August 2019. He demonstrated how his company’s iLine F microscopy system combines sensitive optical capability with machine learning to monitor chimeric antigen receptor (CAR) T-cell expansion, transduction, and activation. The device captures holographic images of cells as they pass through a single-use conduit running between a microscope and bioreactor. Cells then funnel back into culture without additional…
Flow Monitoring in Continuous Processing and Single-Use Systems
Flow sensors placed at critical points in both upstream and downstream processes fulfill the regulatory goals of the process analytical technology (PAT) framework. PAT has been defined as a mechanism for design, analysis, and control of biotechnical and pharmaceutical manufacturing processes through measurement of critical process parameters (CPP). Constant flow monitoring can support its overall targets fundamentally to reduce production cycling time prevent rejection of batches enable real-time release increase automation and control improve energy and material use facilitate continuous…
New Features for Single-Use Pumps in Biopharmaceutical Manufacturing
Everybody has heard the axiom “time is money,” which highlights the belief that time is a valuable resource; therefore, it is better to do things as quickly as possible in order to make more money. This concept perfectly describes the current atmosphere within the biopharmaceutical industry, where drug manufacturers are struggling to overcome speed-to-market challenges in order to reap the financial benefits of an optimized patent window. In order to bring their products to market, most biopharmaceutical-manufacturing systems employ a…
Streamlining the Path to Clinical Manufacturing
Careful consideration of lot size is crucial for multiyear success of a cell therapy business. RoosterBio’s product design and acceleration business works with the company’s customers to help make plans that are appropriate for their stages of product and clinical development. We use the following major considerations in creating a sound multiyear strategy with intermediate milestones: Understand what the future looks like and work backward Use reasonable to conservative assumptions to estimate a range of needs Invest in the right…
Rapid Mammalian Cell Harvest Without Centrifugation for Antibody Purification: Using a Novel System for Cell Culture Media Clarification
Monoclonal antibody (MAb) expression systems typically use signal peptides to ensure secretion of antibodies into cell culture media. Although that reduces the complexity of purification and prevents the need for cell disruption, it does require using expensive and time-consuming techniques to separate cells from antibody-containing cell culture fluids. In this study, we describe our tests of the novel Sartoclear Dynamics Lab V system (Sartorius S Lab Instruments GmbH and Co. KG) for rapid clarification of cell culture media without requiring…
Special Report: Current Analytical Approaches to Biophysical Characterization in a Regulatory Environment
Structural integrity of protein-based therapeutics is one of the major challenges in the biopharmaceutical industry. Multiple factors such as product stability, efficacy, and shelf life could be affected following minor changes in manufacturing process. Multiple biophysical methods employing spectroscopic and calorimetric tools can be used to analyse Higher Order Structure (HOS). Moreover, with an increasing demand for generating as much structural information as possible for regulatory submissions, a requirement for these analyses in a GMP environment is also important. This…
Rapid Implementation of Novel Affinity Purification: Manufacture of Commercial-Scale Next-Generation Antibody Therapies
The rapid and cost-effective production of conventional monoclonal antibodies (MAbs) for clinical trials and commercial supply has contributed toward their wide adoption. Production processes have become more efficient because common purification processes are being used across structurally similar MAbs during key steps of process development and manufacturing. Such successful platform approaches can remove unwanted impurities and are stable across processing conditions, irrespective of the MAb being purified. In addition, they are readily available at the required volume to support large-scale…
Using Slope Spectroscopy for Faster, Repeatable, More Accurate Protein Concentration Measurements
This webcast features: Joe Ferraiolo, Bioanalytics Applications Manager, Repligen Traditional capture chromatography titer measurements that involve dilution requirements represent the highest single source of error in traditional UV analysis. Slope spectroscopy requires no sample preparation or dilution, saving substantial time without changing any other aspects of the assay. This webinar will demonstrate how variable pathlength slope spectroscopy using the SoloVPE™ device can significantly reduce sample prep and process time, while maintaining high repeatability and accuracy. Watch the recorded webcast below.
Highly Sensitive Host Cell Protein Analysis Using µPAC™ LC-MS
This webcast features: Dr. Koen Sandra, Scientific Director, Research Institute for Chromatography, and Geert Van Raemdonck, Global Field Support Expert, PharmaFluidics Within a rapidly growing biopharmaceutical market, there is an increasing demand for the development of a rising number of biotherapeutic protein drugs, such as monoclonal antibodies (MAbs). During the development and production of biotherapeutic proteins, typically using mammalian cell lines, such as Chinese hamster ovary (CHO) cells, additional proteins are expressed as well by the host cell. These so-called…