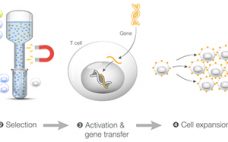
Successful processing and manufacturing in cell and gene therapy workflows are essential to the efficacy of the product. Autologous cell and gene therapy workflows involve isolating cells from an individual, engineering the cells, expanding and concentrating them, and infusing them back into the patient. Certain steps in these workflows could benefit from optimized automation to decrease hands-on time and the cost of the cell manufacturing process. In this application note, we present the Gibco™ Cell Therapy Systems™ (CTS™) Rotea™ Counterflow…
Sponsored Content
Evaluation Of a New Glucose Control Strategy Using CITSens Bio APC (Automated Process Control) for CHO Fed-Batch Application
For the cultivation of cells in research and development, high cell densities and reproducibility are fundamental aims. For that goal a high degree of automation is favorable to reduce the risk of contamination and deviation between batches. As glucose is one of the main energy sources for cells, it is crucial to maintain a constant and controlled automated glucose supply. Therefore, the CITSens Bio APC a combined system to measure and control the glucose concentration in cell culture applications was…
Webcast: Enabling Digital Chromatogram Review for a Faster and More Reliable Operation
This webcast features:Â Martin D. Jensen, Senior Engineer, FUJIFILM Diosynth Biotechnologies Chromatogram review is a monitoring method used to verify process performance in packed-bed chromatography processes. By observing key process parameters such as chromatography column outlet conductivity or UV absorbance, it is possible to identify the signs of a poorly packed column, resin degradation, or equipment malfunction. The industry standard practice relies on performing a paper-based qualitative visual comparison of chromatography profiles against a reference batch. This approach leads to a…
Do Not Let HCPs Delay Your Biologics Route to Market
The stakes are high in biologics development, especially in host cell protein (HCP) analysis. Regulatory bodies have rules and suggested strategies for HCP analysis and reporting, and it is likely that removing HCPs is a key concern in your process development. Ultimately, the accuracy of monitoring and success of purifying your biologic of these HCPs can make or break your product. Get it wrong and it could delay your development cycle. In this blog, we outline strategies to optimize your…
Ensuring Everything is A-OK at AKBA
Accurately and reliably producing pharmaceuticals and biotherapeutics is one of the most important – and challenging – tasks in the world of manufacturing. Any misstep in the production process will lead to the creation of a substandard end product that can do substantial harm to the user. That’s why the processes used in these production chains – things like inline buffer formulation (IBF) and virus filtration – must be completed with no deviation from accepted norms. For Asahi Kasei Bioprocess…
Strategic Development of Characterization and Quality Control Programs for Therapeutic Peptides
This webcast features: Ashleigh Wake, UK Business Development Director, Intertek Pharmaceutical Services Peptide therapeutics are a unique class of pharmaceuticals that may be regarded, in regulatory terms, as either conventional chemical molecules, biological entities or biosimilars. Slight changes in the structure, physicochemical properties, stability, and impurity profile of a peptide can provoke an adverse immune response; therefore, safety assessment is critical. Building a well-thought-out quality control (QC) strategy is key to meeting development milestones and complying with evolving regulatory requirements.…
Predicting Viral Clearance Using a Non-Infectious MVM Surrogate in Downstream Development
This webcast features: David Cetlin, Senior Director, R&D, Cygnus Technologies As viruses can arise during the manufacture of biopharmaceuticals, regulatory agencies require viral clearance validation studies for each biopharmaceutical prior to approval. These studies are typically conducted in biosafety level (BSL)-2 facilities and require large capital and human resources. Due to these hurdles, process knowledge pertaining to viral clearance is limited during development and characterization. The use of an accurate, economical, and quantifiable non-infectious viral surrogate would enable downstream purification…
Updating the Economics of Biologics Manufacturing with 5,000-L Single-Use Bioreactors: A Paradigm Shift
Single-use technologies enable a flexibility and modularity effectively unattainable with more traditional stainless-steel technologies, particularly in upstream bioprocesses. Single-use bioreactors up to 2,000 L are employed largely in preclinical- and clinical-stage bioprocesses to leverage this flexibility. As products reach commercial maturity, scales larger than 2,000 L frequently become desirable to take advantage of economies of scale. With the typical upper limit of single-use bioreactors at 2,000 L, this has traditionally meant transfer to stainless-steel systems. The introduction of the Thermo…
Application-Specific Enhancements to Thermo Fisher Scientific™ HyPerforma™ Single-Use Bioreactors
This webcast features: Ben Madsen, Engineer, Thermo Fisher Scientific The rapid growth of biotherapeutic manufacturing has created significant demand for workflow solutions featuring greater product yield, lower production costs, and accelerated development timelines. To address these demands, developers have moved away from “one-size-fits-all” approaches and are increasingly focused on solutions that address the specific needs of diverse bioproduction processes. Given this shift toward process-specific solutions, Thermo Fisher Scientific™ has introduced a series of application-specific enhancements to the HyPerforma™ Single Use…
To Compress or Not to Compress: What You Need to Know About Packing Resins at Process Scale
This webcast features: Dr. Mark A. Snyder, Manager, Process Chromatography, R&D Applications Group, Bio-Rad Laboratories Join our expert, Dr. Mark A. Snyder, to learn best practices and guidelines for successfully packing different types of chromatography resins. This includes incompressible resins like CHT Ceramic Hydroxyapatite Media as well as traditional compressible resins. CHT is an easy-to-use mixed-mode media and has high specific gravity, a rapid settling rate, and sensitivity to mechanical shear. These variations from traditional compressible resins have to be…