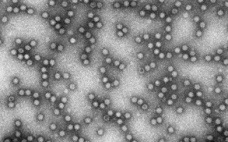
Until recently, downstream process development (PD) teams have lacked methods for easy, effective, and economical estimation of a process’s viral clearance capability. David Cetlin (senior director of R&D at Cygnus Technologies) delivered an “Ask the Expert” presentation on 13 October 2020 describing how MockV kits containing noninfectious mock-virus particles (MVPs) could fill that gap. Cetlin’s Presentation Viral clearance studies tend to be outsourced to contract research organizations (CROs) because they require biosafety level (BSL)-2 and -3 facilities for working with…
Sponsored Content
AAV Suspension Platform Generations: Continuous Improvement Leading to Increased Viral Vector Titer and Yield
This webcast features: Yiyu Dong, Head of Cell Line Development, and David Barnard, Senior Scientist, Process and Technology Development, WuXi Advanced Therapies The field of gene therapy has experienced significant growth in recent years. Adenoassociated virus (AAV)–mediated therapies account for ~70% of the gene therapy market. However, the manufacturing capacity for AAV vectors remains a critical bottleneck. WuXi Advanced Therapies launched a world-class AAV suspension platform early 2020. In this webinar, we will reveal the improvements that increased viral vector…
Enabling Vaccine Production: Solving Challenges
Vaccine developers are in need of more efficient and cost-effective approaches to manufacturing. To achieve this, we actively collaborate with academia, researchers and manufacturers to develop and optimize innovative tools, processes and strategies to resolve bottlenecks and accelerate the availability of vaccines to the global population. This e-book contains a series of case studies highlighting our recent collaborations with organizations and thought leaders on the front lines of the battle against challenging pathogens. From proof-of-concept to full commercial-scale manufacturing, discover…
Exploring New and Improved Analytical Methods for Traditional and Unique Modalities
This webcast features: Jason Sterling, Principal Scientist and Project Director, Analytical and Formulation Resources, and John Rockwell, Group Leader, Catalent Pharma Solutions Biophysical characterization is critical to understand the make-up and behaviors of biologic therapies and vaccines both early in development and throughout the manufacturing scale-up process. As biologics become more complex in structure, and as scientists improve their understanding of the effects of structure on stability, efficacy, safety, etc., there is a need to develop new and improved analytical…
Case Study for a Facility-Fit Driven Process Development
Time to clinic and time to market are the key drivers for client success in the biopharmaceutical industry. Facility fit is becoming key to understanding process constraints and which aspects of the process have the largest impact on enabling facility fit. Process development with facility-fit constraints in mind will ensure a smooth technology transfer and shorten the timeline of current good manufacturing practice (CGMP) product delivery to clients. Fill out the form below to read this Special Report and learn…
Exploring Resin Modalities for Biotherapeutic Purification
New chromatography supports must demonstrate improved selectivity, and bead technologies must be optimized for high binding capacity and product recovery. Drug manufacturers also need access to expertise and continued support from chromatography suppliers that can assist with method development and design of experiments (DoE) assessments. Working together, these industry groups can accelerate method development, increase process yield, reduce buffer consumption, minimize the number of unit operations, and improve overall process economies. Learn more in this Special Report about how resin…
Single-Use Systems: Globalizing Best Practices and Technology Specifications
The challenges of multi-national bioprocessing operations are numerous. They include finding highly skilled experts for each site, lack of expertise with single-use systems, contamination risks and redundant efforts and resources—all of which can lead to higher costs. But these challenges also present opportunities for increasing speed to market, eliminating redundant work and overall cost savings. This white paper details the best practices used in global drug manufacturing. The key is global coordination. Best practices and specifications need to be identified…
Analytical Ultracentrifugation for Characterization of AAV Gene Delivery Vectors
This webcast features: Christopher Sucato, PhD, Associate Director, Biophysical Characterization, and Cynthia Swanson, Associate Research Scientist, Charles River Laboratories Analytical ultracentrifugation (AUC) has been a staple in the biopharmaceutical industry to analyze aggregation and higher-order structure in protein drug products. With the recent boom in cell and gene therapies using gene-delivery vectors, new avenues for AUC-based characterization and QC lot release methodologies are now available. View this webinar for a discussion on the new parameters of AUC analysis and how…
One-step isolation and activation of naïve and early memory T cells with CTS Dynabeads CD3/CD28
This application note demonstrates the near 100% recovery of naïve and early memory T cells using Gibco™ CTS™ Dynabeads™ CD3/CD28 beads. In a one-step process, CTS Dynabeads CD3/CD28 simultaneously isolates and activates T cells uniformly. Find out more about this one-step process. 
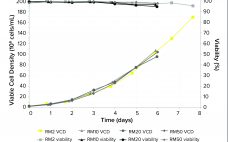
Intensified Seed Train Strategy for Faster, Cost-Effective Scale-Up of Biologics Manufacturing
The high costs of and limits on global accessibility of biologics such as monoclonal antibodies (MAbs) are focusing the biopharmaceutical industry’s attention on strategies for rapid, economical development of such therapies. Process intensification is one approach to help shorten manufacturing timelines and reduce cost of goods (CoG) (1, 2). Today, process intensification in upstream cell culture enables biologics manufacturing in facilities with smaller footprints and lower scale-up volumes than was possible before. Intensified processing of Chinese hamster ovary (CHO) clones…