This webcast features: Oliver Berteau, Pharmaceutical & Biotech Industry Manager, METTLER TOLEDO Process Analytics Inline dissolved CO2 (dCO2) measurement and monitoring during cell culture offers significant benefits for process control impacting productivity and quality attributes. Real-time dCO2 measurement complements routine dissolved CO2 measurements with a blood gas analyzer (BGA) during preclinical and commercial operations. Animal and insect cells are more or less sensitive to the level of dCO2 during cell culture. Toxic below and beyond specific thresholds, dCO2 is also…
Sponsored Content
The Critical Steps for Protein Therapeutic Potency Assay Development
This webcast features: Jennifer Lawson, PhD, Global Product Manager, Cell Banking and Testing, Sartorius Potency assays are an important part of the drug development process and are required throughout the lifetime of the product. The potency assay needs to correlate with the mechanism of action and provide an indication of stability. With these requirements comes a myriad of challenges in the development process, especially as therapeutics become increasingly complex. Assay development should be stepwise, starting with proof of concept and…
HCP Analysis by ELISA and Orthogonal Methods in Vaccine and Gene Therapy Development
This webcast features: Jared Isaac, PhD, Sr. Scientist, Chromatography and Mass Spectrometry, Cygnus Technologies Next-generation recombinant vaccines and gene therapy products require clinical and commercial manufacturing of protein antigens or viral vectors produced using cell culture technologies. Regulatory guidelines require testing for cell substrate related impurities, media and purification additives, as well as adventitious agents throughout vaccine and gene therapy development to study the candidate’s purity, safety, and efficacy. While low levels of most impurities can be inconsequential, patient safety…
Autologous CAR T Cell Manufacturing Using a Semiautomatic, Closed, Modular Workflow
Cell-based chimeric antigen receptor (CAR) T cell therapies have rapidly advanced in recent years, with a variety of targets in clinical research and several FDA approved products already on the market. There has been tremendous effort to make CAR T cells more effective, safe, and persistent when treating patients. On the manufacturing side, however, errors, lot-to-lot variation, and contamination can be associated with open processes and manual handling of CAR T cells. Cell isolation, gene editing, expansion, and cryopreservation are…
A Complete Solution for MSC Therapy Workflows: Cell Scale-Up, Cryopreservation, and DMSO Removal
Mesenchymal stem cells (MSCs) are used frequently for cell therapy applications. As multipotent cells, they can differentiate into other lineages such as adipocytes, osteocytes, and chondrocytes. Additionally, they are known to secrete trophic factors that can play important roles in immunoregulation. Although MSCs can be isolated from several different tissue sources, those derived from bone marrow commonly are studied because they are easy to access in quantities large enough for therapeutic dosing (2 Ă— 106 cells/kg of body weight). Still,…
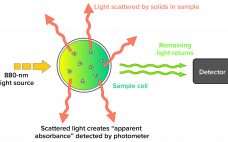
Validation of a Next-Generation Single-Use Turbidity System
Turbidity describes the relative clarity of a liquid as the result of suspended solids. Instruments that measure turbidity typically use a beam of light to detect particles by measuring the difference between the amount of light emitted from the light source and the amount that is received by a detector. Such measurements are affected by the size, shape, and number of particles in a sample of liquid because those solids scatter the incoming light, which provides an apparent absorbance that…
Overcoming Obstacles in AAV Viral Vector Manufacturing
Rapidly growing interest in gene therapy has led to the need for more cost-effective and scalable viral-vector manufacturing platforms. Adenoassociated virus (AAV) has become a vector of choice because of its safety profile (nonpathogenic infection). In addition, AAV cannot replicate on its own and is not integrated directly into the host genome. AAV vector manufacturing using human embryonic kidney (HEK) cells in either adherent or suspension mode includes several typical processing steps: cell expansion, plasmid transfection, viral-vector production, cell lysis,…
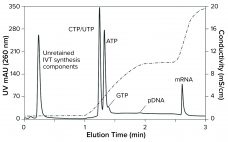
Eliminating the Analytical Bottleneck in Production and Purification of mRNA
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval (1). That same technology can hasten development and approval of other therapeutic classes, including cancer immunotherapy, protein replacement, and gene therapy. Fulfilling those opportunities imposes significant challenges on process developers and manufacturers to improve existing processes. Scale-up to produce millions of doses (tens of kilograms) compounds those challenges. Furthermore, every step of the journey requires high-performance analytical methods, to ensure patient safety and…
Edata Exchange – Embark On Your Digital Transformation Journey
You need the right data at the right time from your supplier to make important production and supply chain decisions to achieve your Biopharma Manufacturing goals. Now you have the eMERGE™ Program, our standard platform for the exchange of electronic data. It provides key raw material data, electronically, for every shipment, so you can start making informed decisions right away.
Vero Cell-based Vaccine Production: Cell lines, Media and Bioreactor Options
The recent Covid-19 pandemic introduced new challenges for the vaccine industry, it has also brought in new innovations in vaccine development including DNA/RNA based vaccines. The pandemic also increased the demand for well-established cell-based vaccine production technologies. The article reviews strategies for optimizing Vero cell-based vaccine production using rabies and influenza as examples. The Vero cell line is one of the most satisfactory vaccine production hosts based on its infectability, stability and well-documented performance in quality and quantity of viral…